PF024
MMP-9, Active, Human, Recombinant
Sinonimo/i:
Gelatinase B, 83 kDa Gelatinase, Matrix Metalloproteinase 9, Matrix Metalloproteinase 9, Gelatinase B, 83 kDa Gelatinase
About This Item
Prodotti consigliati
Saggio
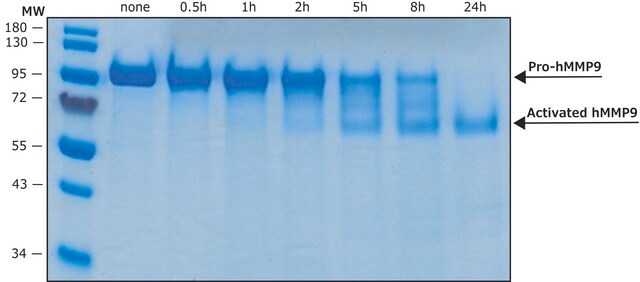
≥90% (SDS-PAGE)
Livello qualitativo
Forma fisica
liquid
Attività specifica
≥8.0 ΔA405/h-μg protein (thiopeptide hydrolysis assay)
non contiene
preservative
Produttore/marchio commerciale
Calbiochem®
Condizioni di stoccaggio
OK to freeze
avoid repeated freeze/thaw cycles
Condizioni di spedizione
wet ice
Temperatura di conservazione
−70°C
Descrizione generale
Regulation of MMP activity can occur at the level of gene expression, including transcription and translation, level of activation, or at the level of inhibition by TIMPs. Thus, perturbations at any of these points can theoretically lead to alterations in ECM turnover. Expression is under tight control by pro- and anti-inflammatory cytokines and/or growth factors and, once produced the enzymes are usually secreted as inactive zymograms. Upon activation (removal of the inhibitory propeptide region of the molecules) MMPs are subject to control by locally produced TIMPs. All MMPs can be activated in vitro with organomercurial compounds (e.g. 4-aminophenylmercuric acetate), but the agents responsible for the physiological activation of all MMPs have not been clearly defined. Numerous studies indicate that members of the MMP family have the ability to activate one another. The activation of the MMPs in vivo is likely to be a critical step in terms of their biological behavior, because it is this activation that will tip the balance in favor of ECM degradation. The hallmark of diseases involving MMPs appear to be stoichiometric imbalance between active MMPs and TIMPs, leading to excessive tissue disruption and often degradation. Determination of the mechanisms that control this imbalance may open up some important therapeutic options of specific enzyme inhibitors.
Confezionamento
Attenzione
Stato fisico
Ricostituzione
Altre note
Backstrom, J.R., et al. 1996. J. Neuro.16, 7910.
Lim, G.P., et al. 1996. J. Neurochem.67, 251.
Xia, T., et al. 1996. Biochim. Biophys. Acta1293, 259.
Sang, Q.X., et al. 1995. Biochim. Biophys. Acta1251, 99.
Zempo, N., et al. 1994. J. Vasc. Surg.20, 217.
Birkedal-Hansen, H. 1993. J. Periodontol.64, 484.
Stetler-Stevenson, W.G., et al. 1993. FASEB J.7, 1434.
Jeffrey, J.J. 1991. Semin. Perinatol.15, 118.
Liotta, L.A., et al. 1991. Cell64, 327.
Harris, E. 1990. N. Engl. J. Med.322, 1277.
Note legali
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.