Fontos dokumentumok
901824
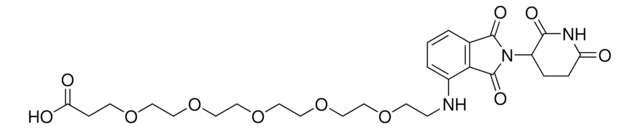
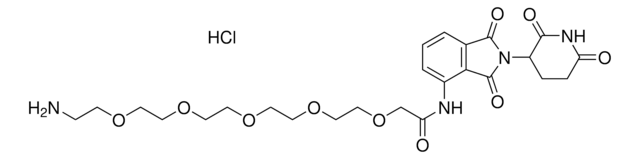
Pomalidomide-PEG4-CO2H
Szinonimák:
1-((2-(2,6-Dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)-3,6,9,12-tetraoxapentadecan-15-oic acid, Crosslinker–E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Javasolt termékek
ligand
pomalidomide
form
solid
reakcióalkalmasság
reactivity: amine reactive
reagent type: ligand-linker conjugate
funkcionális csoport
carboxylic acid
tárolási hőmérséklet
2-8°C
SMILES string
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NCCOCCOCCOCCOCCC(O)=O)=O)NC1=O
Related Categories
Alkalmazás
Automate your CRBN-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
Egyéb megjegyzések
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Development of Highly Potent and Selective Steroidal Inhibitors and Degraders of CDK8
Jogi információk
kapcsolódó termék
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Cikkek
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással