Fontos dokumentumok
901503
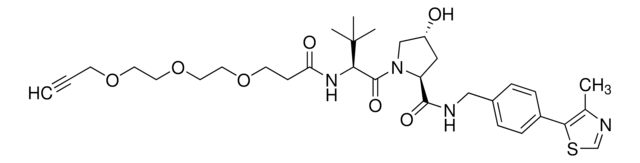
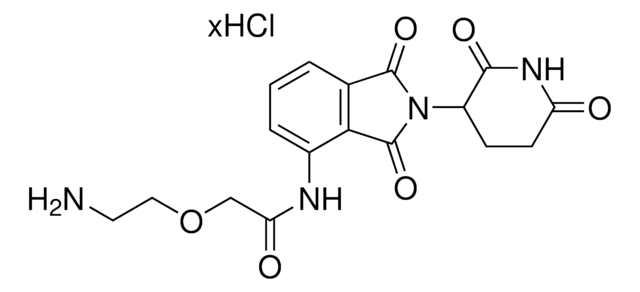
(S,R,S)-AHPC-PEG1-Alkyne
≥95%
Szinonimák:
(2S,4R)-1-((S)-3,3-Dimethyl-2-(3-(prop-2-yn-1-yloxy)propanamido)butanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, CrosslinkerE3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
Javasolt termékek
ligand
VH032
Minőségi szint
Teszt
≥95%
form
powder or crystals
reakcióalkalmasság
reaction type: click chemistry
reagent type: ligand-linker conjugate
funkcionális csoport
alkyne
tárolási hőmérséklet
2-8°C
SMILES string
O=C(NCC1=CC=C(C2=C(C)N=CS2)C=C1)[C@H](C[C@@H](O)C3)N3C([C@H](C(C)(C)C)NC(CCOCC#C)=O)=O
Alkalmazás
Automate your VHL-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
Egyéb megjegyzések
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Jogi információk
kapcsolódó termék
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Sajnos jelenleg COA nem áll rendelkezésre ehhez a termékhez online.
Ha segítségre van szüksége, lépjen velünk kapcsolatba Vevőszolgálat
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Cikkek
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással