Fontos dokumentumok
PHR1128
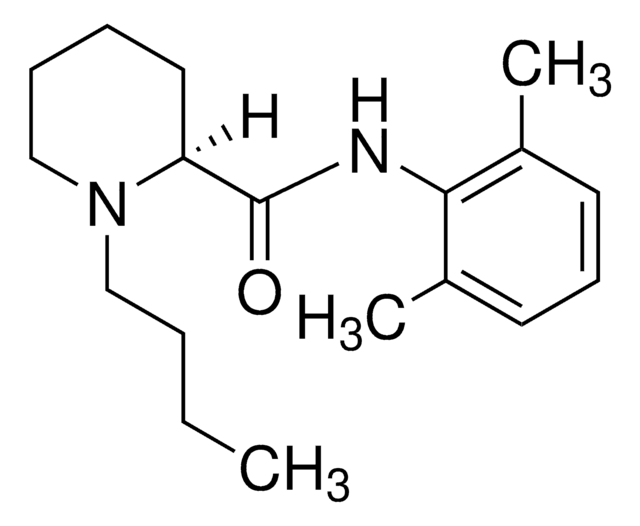
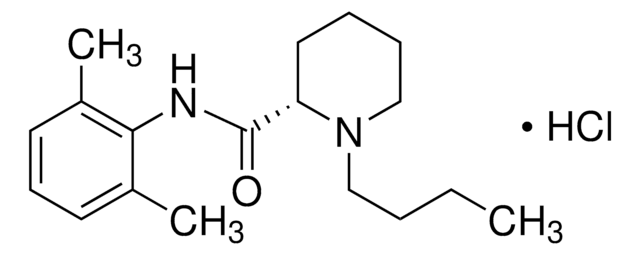
Bupivacaine hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
Szinonimák:
Bupivacaine hydrochloride monohydrate, 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide
About This Item
Javasolt termékek
grade
certified reference material
pharmaceutical secondary standard
Minőségi szint
Ügynökség
traceable to BP 479
traceable to Ph. Eur. B1160000
traceable to USP 1078507
API-család
bupivacaine
Analitikai műbizonylat
current certificate can be downloaded
technika/technikák
HPLC: suitable
gas chromatography (GC): suitable
alkalmazás(ok)
pharmaceutical (small molecule)
format
neat
tárolási hőmérséklet
2-30°C
SMILES string
O.Cl.CCCCN1CCCCC1C(=O)Nc2c(C)cccc2C
InChI
1S/C18H28N2O.ClH.H2O/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3;;/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21);1H;1H2
Nemzetközi kémiai azonosító kulcs
HUCIWBPMHXGLFM-UHFFFAOYSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
Biokémiai/fiziológiai hatások
Analízis megjegyzés
Egyéb megjegyzések
Lábjegyzet
Javasolt termékek
kapcsolódó termék
Figyelmeztetés
Danger
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 2 Oral
Tárolási osztály kódja
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással