905380
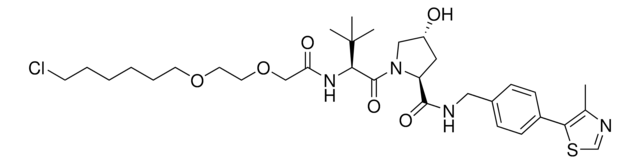
(S,R,S)-AHPC-C6-PEG3-butyl chloride
≥95%
Sinonimo/i:
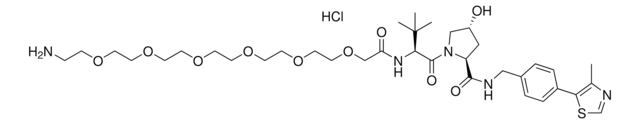
(2S,4R)-1-((S)-2-(tert-Butyl)-22-chloro-4-oxo-10,13,16-trioxa-3-azadocosanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, (S,R,S)-AHPC-6-2-2-6-Cl, Crosslinker−E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
Scegli un formato
354,20 €
Prezzo di listino506,00 €Risparmia il 30%Scegli un formato
About This Item
354,20 €
Prezzo di listino506,00 €Risparmia il 30%Prodotti consigliati
ligand
VH032
Saggio
≥95%
Stato
solid
Impiego in reazioni chimiche
reactivity: sulfuryl reactive
reagent type: ligand-linker conjugate
Gruppo funzionale
alkyl halide
Temperatura di conservazione
2-8°C
Stringa SMILE
ClCCCCCCOCCOCCOCCCCCC(N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C)=O
InChI
1S/C38H59ClN4O7S/c1-28-34(51-27-41-28)30-15-13-29(14-16-30)25-40-36(46)32-24-31(44)26-43(32)37(47)35(38(2,3)4)42-33(45)12-8-7-11-19-49-21-23-50-22-20-48-18-10-6-5-9-17-39/h13-16,27,31-32,35,44H,5-12,17-26H2,1-4H3,(H,40,46)(H,42,45)/t31-,32+,35-/m1/s1
MLRLOIWXHCPWFF-MEEYNGGZSA-N
Categorie correlate
Applicazioni
Altre note
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Note legali
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.