909378
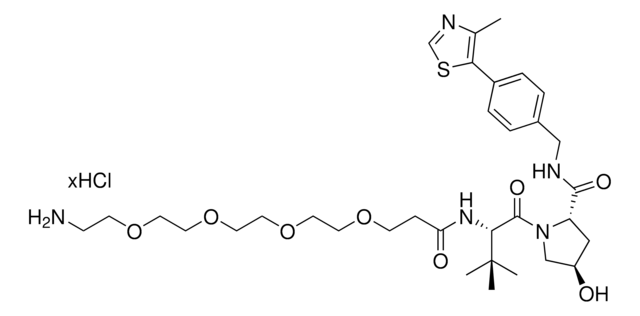
(S,R,S)-AHPC-PEG6-Azide
≥95%
Sinônimo(s):
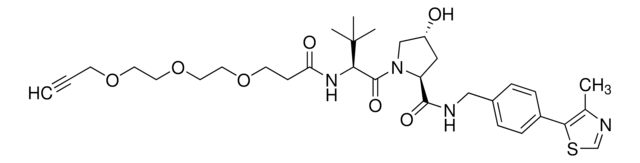
(2S,4R)-1-((S)-23-Azido-2-(tert-butyl)-4-oxo-6,9,12,15,18,21-hexaoxa-3-azatricosanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, Crosslinker-E3 ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
Selecione um tamanho
Selecione um tamanho
About This Item
Produtos recomendados
ligand
VH032
Ensaio
≥95%
Formulário
(Liquid or Semi-Solid or Paste or Solid)
adequação da reação
reaction type: click chemistry
reagent type: ligand-linker conjugate
grupo funcional
azide
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
O=C(COCCOCCOCCOCCOCCOCCN=[N+]=[N-])N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C
InChI
1S/C36H55N7O10S/c1-26-32(54-25-39-26)28-7-5-27(6-8-28)22-38-34(46)30-21-29(44)23-43(30)35(47)33(36(2,3)4)41-31(45)24-53-20-19-52-18-17-51-16-15-50-14-13-49-12-11-48-10-9-40-42-37/h5-8,25,29-30,33,44H,9-24H2,1-4H3,(H,38,46)(H,41,45)/t29-,30+,33-/m1/s1
chave InChI
AJSBEOXYBAZQFR-YLJHYDRVSA-N
Categorias relacionadas
Aplicação
Automate your VHL-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
Outras notas
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Informações legais
produto relacionado
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Active Filters
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica




![(1S,4S,8S)-5-Benzyl-2-isobutyl-8-methoxy-1,8-dimethylbicyclo[2.2.2]octa-2,5-diene 97%](/deepweb/assets/sigmaaldrich/product/structures/145/868/298d62ec-c66e-41b7-999a-56634852a68f/640/298d62ec-c66e-41b7-999a-56634852a68f.png)

