After administration by the mouth, the half-life is 2 to 5 hours 1. In plasma, about 92-98% binds to plasma proteins. Nifedipine is completely metabolized. About 70% of a dose is excreted in the urine in 24 hours as metabolites including 5-methoxycarbonyl-2,6-dimethyl-4-(2-nitrophenyl) pyridine-3-carboxylic acid; dimethyl 2,6-dimethyl-4-(2-nitrophenyl pyridine-3,5-dicarboxylate and 2-hydroxymethyl-5-methoxycarbonyl-6-methyl-4-(2-nitrophenyl) pyridine-3-carboxylic acid and its lactone derivative. Up to 15% of a dose is eliminated in the feces as metabolites in 4 days 2. References: 1. Martindale, 29th ed., pgs. 1509-1513. 2. Clarke's Isolation and Identification of Drugs., 2nd ed., p. 811.
Kluczowe dokumenty
N7634
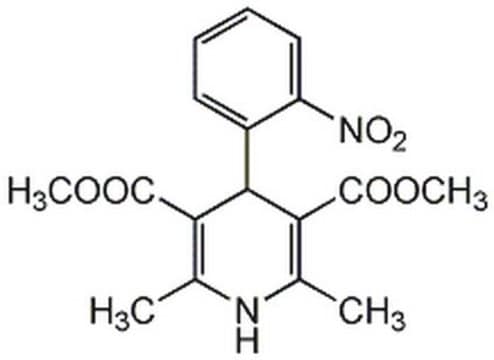
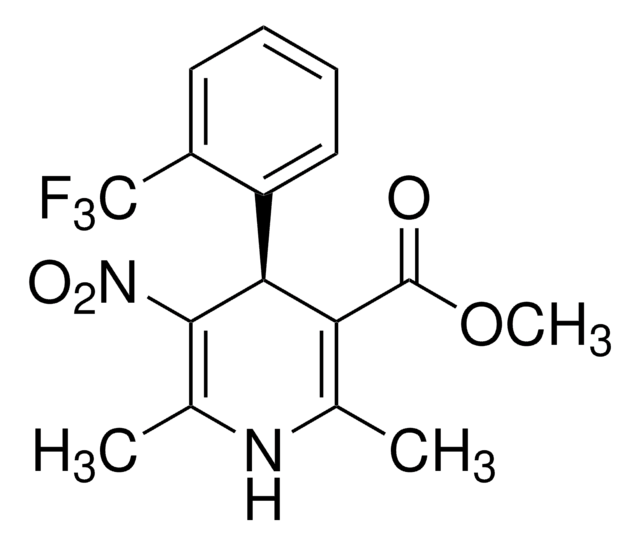
Nifedipine
≥98% (HPLC), powder, L-type Ca²⁺ channel blocker
Synonim(y):
1,4-Dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid dimethyl ester
Wybierz wielkość
405,00 zł
Wybierz wielkość
About This Item
405,00 zł
Polecane produkty
Nazwa produktu
Nifedipine, ≥98% (HPLC), powder
Próba
≥98% (HPLC)
Formularz
powder
kolor
yellow
rozpuszczalność
DMSO: soluble
ethanol: soluble
inicjator
Bayer
temp. przechowywania
2-8°C
ciąg SMILES
COC(=O)C1=C(C)NC(C)=C(C1c2ccccc2[N+]([O-])=O)C(=O)OC
InChI
1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
Klucz InChI
HYIMSNHJOBLJNT-UHFFFAOYSA-N
informacje o genach
human ... ADORA2A(135) , ADORA3(140) , CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779) , CACNA2D1(781) , CYP1A2(1544) , KCNH1(3756) , TTR(7276)
mouse ... Cacna1c(12288)
rat ... Adora1(29290) , Adora2a(25369) , Cacna1c(24239) , Cacna1d(29716) , Kcnj1(24521) , Kcnn4(65206) , Tbxas1(24886)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- to evaluate its effect on myenteric neuronal calcium current through R-type calcium channel in guinea pig small intestine[1]
- to evaluate the neuroprotective effect of L-type calcium channel blockers in cholinergic and dopaminergic neurons[2]
- to identify the effect of co-administration of nifedipine (anti-hypertensive drug) along with hypoglycemic drug on human umbilical vein cells (HUVECs)[3]
Działania biochem./fizjol.
Cechy i korzyści
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Discover Bioactive Small Molecules for ADME/Tox
-
What is the half life of Product N7634, Nifidipine, in vivo?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the solubility of Product N7634, Nifidipine?
1 answer-
Nifedipine can be dissolved in DMSO at 50 mg/ml1. It is sparingly soluble in absolute ethanol2. Herembert, T. et al., dissolved nifedipine in absolute ethanol (no concentration reported); the maximum ethanol concentration in cultures was 0.2% without any effect of solvent on the cells3. Nifedipine is soluble (g/L, at 20°C) in the following solvents: acetone, 250; methylene chloride, 160; chloroform, 140; ethyl acetate, 50; methanol, 26; ethanol, 17.4 It is practically insoluble in water. The solubilities at 37°C in buffer solutions of different pH values are: pH 4, 0.0058 g/L; pH 7, 0.0056 g/L; pH 9.0, 0.0078 g/L; pH 13, 0.006 g/L1. References: 1. Ali, S.L., Analytical Profiles of Drug Substances, 18, 221, (1989). 2. Martindale, The Extra Pharmacopoeia, 30th ed., 374, (1993). and 3. Herembert, T. et al. Brit. J. Pharmacol., 114, 1703, (1995).
Helpful?
-
-
Are there any recommended dosages for Product N7634, Nifidipine, in vivo and in vitro?
1 answer-
Nifedipine is reported to inhibit Ca2+-sensitive K+ channels at 100 μM1. Doses for different animals have been reported2,3. In randomly growing cultures of aortic cells of rats, nifedipine at 10 μM inhibited cell proliferation. References: 1. Thomas-Young, R.J. et al. Biochim. Biophys. Acta, 1146, 81, (1993). 2. Drug Dosages In Laboratory Animals: A Handbook, 3rd ed., Telford Press. and 3. Borchard, R.E. et al. Drug Dosage in Laboratory Animals, A Handbook, Third Edition, p. 315, The Telford Press, (1990).
Helpful?
-
-
Are there any recommended conditions that Product N7634, Nifidipine, should be used in?
1 answer-
When exposed to daylight and certain wavelengths of artificial light, nifedipine readily converts to a nitrosophenylpyridine derivative. Exposure to ultra-violet light leads to the formation of a nitrophenylpyridine derivative. Therefore, USP recommends that assays be performed in the dark or under golden fluorescent or other low actinic light. Low actinic glassware should be used1.
Helpful?
-
Active Filters
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej