Fontos dokumentumok
N3503
Nystatin
≥4,400 USP units/mg
Szinonimák:
Fungicidin, Mycostatin
About This Item
Javasolt termékek
form
powder
Minőségi szint
specifikus aktivitás
≥4,400 USP units/mg
oldhatóság
water: insoluble
antibiotikus hatásspektrum
fungi
yeast
Hatásmechanizmus
cell membrane | interferes
tárolási hőmérséklet
−20°C
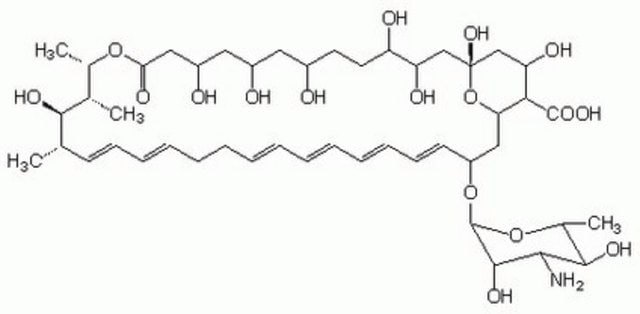
SMILES string
O[C@@H]([C@H](C)[C@H](C)O1)[C@@H](C)/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@]3([H])[C@H](C(O)=O)[C@@H](O)C[C@](O)(O3)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)C[C@@H](O)CC1=O
InChI
1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28+,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1
Nemzetközi kémiai azonosító kulcs
VQOXZBDYSJBXMA-QEKUPDCNSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Related Categories
Általános leírás
Chemical structure: polyene
Alkalmazás
Biokémiai/fiziológiai hatások
Antimicrobial spectrum: Nystatin acts against fungi, yeasts and molds.
Vigyázat
Elkészítési megjegyzés
Egyéb megjegyzések
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással