Fontos dokumentumok
A5513
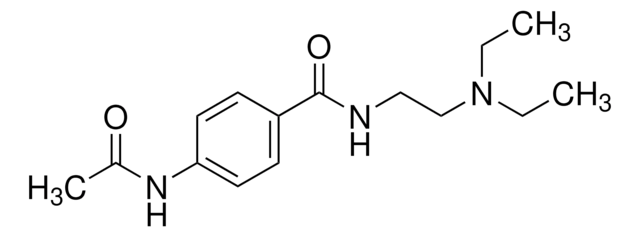
N-Acetylprocainamide hydrochloride
≥99% (HPLC), powder
Szinonimák:
N-Acetylnovocainamide hydrochloride, Acecainide hydrochloride, NAPA
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥99% (HPLC)
Forma
powder
mp
184-186 °C (lit.)
oldhatóság
H2O: 50 mg/mL
tárolási hőmérséklet
−20°C
SMILES string
Cl[H].CCN(CC)CCNC(=O)c1ccc(NC(C)=O)cc1
InChI
1S/C15H23N3O2.ClH/c1-4-18(5-2)11-10-16-15(20)13-6-8-14(9-7-13)17-12(3)19;/h6-9H,4-5,10-11H2,1-3H3,(H,16,20)(H,17,19);1H
Nemzetközi kémiai azonosító kulcs
IYEWBJUCJHKLHD-UHFFFAOYSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Alkalmazás
- as an internal standard for spiking plasma samples for ultra-high-pressure liquid chromatography coupled with a diode array detector (UHPLC-DAD) analysis
- to test its relaxant effect on tracheal smooth muscle tissue preparations
- in preparation of complexes with N-acetyl-L-tyrosine methyl ester and N-acetyl-L-phenylalanine methyl ester for studying intermolecular interactions using nuclear magnetic resonance (NMR) spectroscopy studies
Biokémiai/fiziológiai hatások
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással