Fontos dokumentumok
T2501200
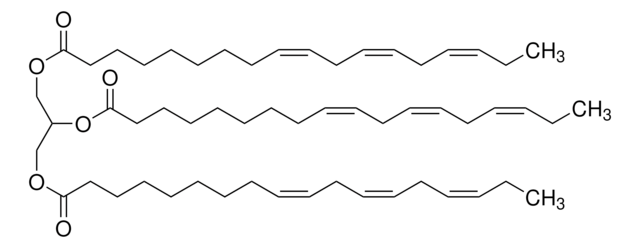
Tristearin
European Pharmacopoeia (EP) Reference Standard
Szinonimák:
Glyceryl tristearate, 1,2,3-Trioctadecanoylglycerol, Glycerol tristearate, Tristearin
About This Item
Javasolt termékek
grade
pharmaceutical primary standard
API-család
tristearin
gyártó/kereskedő neve
EDQM
alkalmazás(ok)
pharmaceutical (small molecule)
Formátum
neat
tárolási hőmérséklet
−20°C
SMILES string
CCCCCCCCCCCCCCCCCC(=O)OCC(COC(=O)CCCCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCCCC
InChI
1S/C57H110O6/c1-4-7-10-13-16-19-22-25-28-31-34-37-40-43-46-49-55(58)61-52-54(63-57(60)51-48-45-42-39-36-33-30-27-24-21-18-15-12-9-6-3)53-62-56(59)50-47-44-41-38-35-32-29-26-23-20-17-14-11-8-5-2/h54H,4-53H2,1-3H3
Nemzetközi kémiai azonosító kulcs
DCXXMTOCNZCJGO-UHFFFAOYSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
Kiszerelés
Egyéb megjegyzések
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 1
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumentumok section.
Ha segítségre van szüksége, lépjen velünk kapcsolatba Vevőszolgálat
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással