Fontos dokumentumok
870450O
Avanti
2-AG
Avanti Research™ - A Croda Brand 870450O
Szinonimák:
2-arachidonoyl glycerol
About This Item
Javasolt termékek
Forma
liquid
kiszerelés
pkg of 1 × 5 mg (with screw cap/argon/foil bag (870450O-5mg))
gyártó/kereskedő neve
Avanti Research™ - A Croda Brand 870450O
lipidtípus
bioactive lipids
phosphoglycerides
kiszállítva
dry ice
tárolási hőmérséklet
−70°C
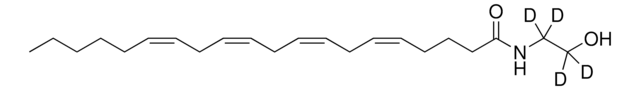
SMILES string
[H]C(CO)(OC(CCC/C=C\C/C=C\C/C=C\C/C=C\CCCCC)=O)CO
InChI
1S/C23H38O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23(26)27-22(20-24)21-25/h6-7,9-10,12-13,15-16,22,24-25H,2-5,8,11,14,17-21H2,1H3/b7-6-,10-9-,13-12-,16-15-
Nemzetközi kémiai azonosító kulcs
RCRCTBLIHCHWDZ-DOFZRALJSA-N
Általános leírás
Kiszerelés
Jogi információk
Tárolási osztály kódja
12 - Non Combustible Liquids
WGK
WGK 3
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumentumok section.
Ha segítségre van szüksége, lépjen velünk kapcsolatba Vevőszolgálat
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással