Fontos dokumentumok
E7521
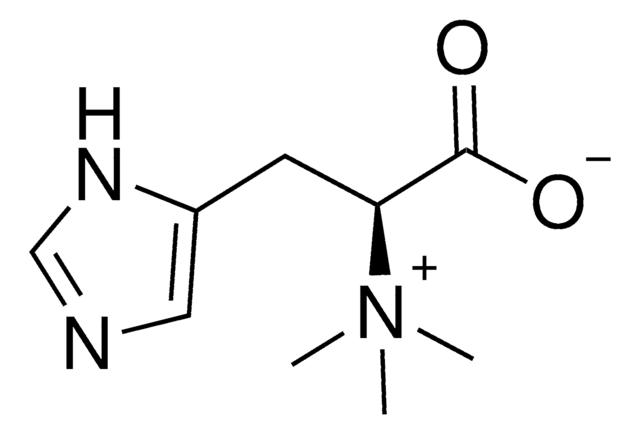
L-(+)-Ergothioneine
Szinonimák:
2-mercaptohistidine trimethyl betaine, Ergothioneine, Sympectothion, Thiasine, Thiolhistidine-betaine, Thioneine, (S)-α-Carboxy-N,N,N-trimethyl-2-mercapto-1H-imidazole-4-ethanaminium inner salt
About This Item
Javasolt termékek
biológiai forrás
fungus (Actinomycetales)
fungus (Ascomycota)
fungus (Basidiomycota)
Minőségi szint
Teszt
≥98.0%
Forma
powder
molekulatömeg
229.30
tárolási körülmény
(Keep container tightly closed in a dry and well-ventilated place)
technika/technikák
protein quantification: suitable
oldhatóság
water: 50 mg/mL, clear, colorless
tárolási hőmérséklet
−20°C
SMILES string
C[N+](C)(C)[C@@H](Cc1c[nH]c(S)n1)C([O-])=O
InChI
1S/C9H15N3O2S/c1-12(2,3)7(8(13)14)4-6-5-10-9(15)11-6/h5,7H,4H2,1-3H3,(H2-,10,11,13,14,15)/t7-/m0/s1
Nemzetközi kémiai azonosító kulcs
SSISHJJTAXXQAX-ZETCQYMHSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Research area: Apoptosis
Alkalmazás
L-(+)-Ergothioneine has been used:
- as a component of the maturation medium for cumulus-oocyte complexes (COCs) to test protective function on lipid peroxide formation
- as an antioxidant compound to test type 2 diabetes patients
- as a positive control in solute carrier protein 22 A4 (SLC22A4) transport assay
Biokémiai/fiziológiai hatások
Kiszerelés
Egyéb megjegyzések
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással