Fontos dokumentumok
699800P
Avanti
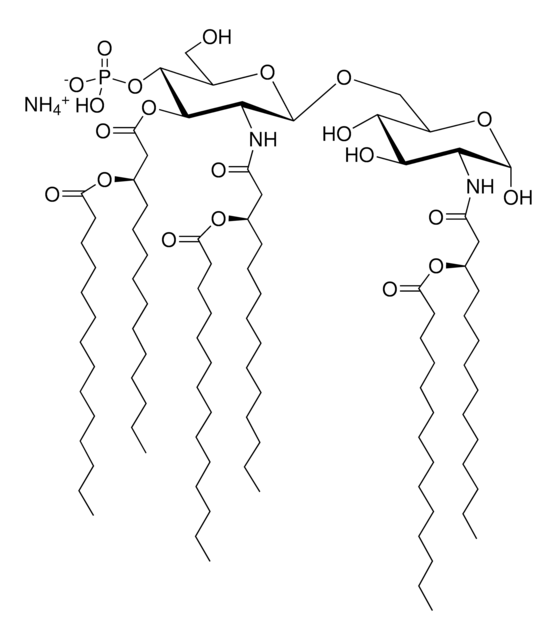
MPLA (PHAD™)
Avanti Research™ - A Croda Brand
Szinonimák:
PHAD™ phosphorylated hexaacyl disaccharide; Glycopyranoside Lipid A; GLA
About This Item
Javasolt termékek
leírás
Monophosphoryl Lipid A (Synthetic) (PHAD™)
Teszt
>99% (HPLC)
form
powder
kiszerelés
pkg of 1 × 1 mg (699800P-1mg)
pkg of 1 × 5 mg (699800P-5mg)
gyártó/kereskedő neve
Avanti Research™ - A Croda Brand
alkalmazás(ok)
vaccine development
kiszállítva
dry ice
tárolási hőmérséklet
−20°C
Általános leírás
Alkalmazás
- as a component of cobalt porphyrin-phospholipid (Co-PoP) liposomes for the immunization of mice with membrane proximal external region (MPER) of the gp41 envelope protein
- as an adjuvant along with dimethyldioctadecylammonium bromide(DDA) for C. muridarum recombinant membrane protein based multi-subunit vaccine
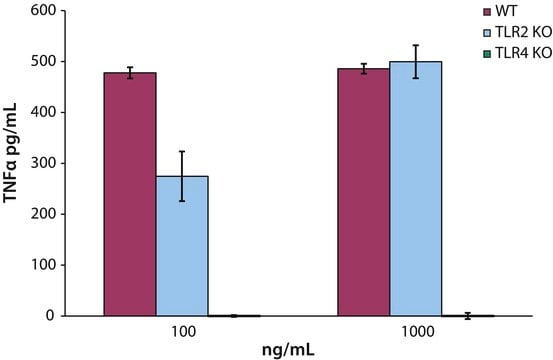
- as toll-like receptor-4 (TLR4) agonist adjuvant for respiratory syncytial virus (RSV) fusion (F) protein FI-RSV vaccine
Biokémiai/fiziológiai hatások
Kiszerelés
Egyéb megjegyzések
Jogi információk
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással