33262
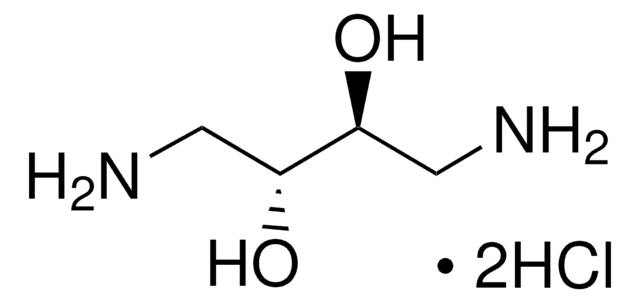
1,3-Diamino-2-propanol
purum, ≥96.5% (GC)
Synonym(s):
1,3-Diamino-2-hydroxypropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CH(OH)CH2NH2
CAS Number:
Molecular Weight:
90.12
Beilstein:
741859
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥96.5% (GC)
form
solid
impurities
≤2.0% water
mp
40-44 °C (lit.)
solubility
water: soluble 1 g/10 mL
storage temp.
2-8°C
SMILES string
NCC(O)CN
InChI
1S/C3H10N2O/c4-1-3(6)2-5/h3,6H,1-2,4-5H2
InChI key
UYBWIEGTWASWSR-UHFFFAOYSA-N
Gene Information
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,3-Diamino-2-propanol was used in the synthesis of poly(2-hydroxypropylene imine), poly(2-hydroxypropylene imine ethylene imine) and poly(hydroxypropylene imine propylene imine). It was also used in the synthesis of tetranuclear mixed ligand copper(II) complex of a pyrazole containing Schiff base and a hydroxyhexahydropyrimidylpyrazole group.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sachindranath Pal et al.
Inorganic chemistry, 44(11), 3880-3889 (2005-05-24)
A tetranuclear mixed ligand copper(II) complex of a pyrazole containing Schiff base and a hydroxyhexahydropyrimidylpyrazole and copper(II) and nickel(II) complexes of the Schiff base having N-donor atoms have been investigated. A 2 equiv amount of 5-methyl-3-formylpyrazole (MPA) and 2 equiv
J M Matés et al.
Biochemical pharmacology, 42(5), 1045-1052 (1991-08-08)
Ornithine decarboxylase (ODC) activity of Ehrlich carcinoma cells was increased more than 36-fold after being maintained for 3.5 hr in vitro in a special chamber which allowed continuous perifusion with 0.5 mM ornithine; if incubated in vitro without perifusion the
L Alhonen-Hongisto et al.
The Biochemical journal, 188(2), 491-501 (1980-05-15)
The anti-proliferative effects of 1,1'-[(methylethanediylidene)dinitrilo]diguanidine [methylglyoxal bis(guanylhydrazone)] and 1,1'-[(metHYLETHANEDIYLIDENE)dinitrilo]bis-(3-aminoguaNIDINE) HAVE BEEN STUDIED IN Ehrlich ascites carcinoma cells grown in suspension cultures. Both compounds are potent inhibitors of S-adenosyl-L-methionine decarboxylase from the tumour cells. In the presence of putrescine (but not
M Mach et al.
The Biochemical journal, 202(1), 153-162 (1982-01-15)
Inhibitors of polyamine synthesis (alpha-methylornithine and 1,3-diaminopropan-2-ol) were used to study the relationship between polyamine synthesis and specific methylations of tRNA in Dictyostelium discoideum during vegetative growth. Polyamine concentrations were found to be 10 mM for putrescine, 1.6 mM for
Y W Hu et al.
Canadian journal of physiology and pharmacology, 60(12), 1493-1498 (1982-12-01)
The effect of diaminopropanol, an inhibitor of polyamine synthesis, on the metabolic response of liver to insulin was studied in streptozotocin-diabetic rats. Insulin elicited a prompt and very marked increase in ornithine and S-adenosylmethionine decarboxylase activities and in putrescine concentration.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service