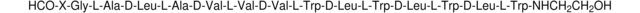Fontos dokumentumok
A4665
Alamethicin from Trichoderma viride
≥98% (HPLC)
Szinonimák:
Antibiotic U-22324
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥98% (HPLC)
Forma
powder
antibiotikus hatásspektrum
Gram-positive bacteria
Hatásmechanizmus
cell membrane | interferes
tárolási hőmérséklet
2-8°C
SMILES string
CC(C)C[C@H](NC(=O)CNC(=O)C(C)(C)NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)C(C)(C)NC(=O)[C@H](C)NC(=O)C(C)(C)NC(=O)[C@@H]1CCCN1C(=O)C(C)(C)NC(C)=O)C(C)C)C(=O)NC(C)(C)C(=O)N2CCC[C@H]2C(=O)N[C@@H](C(C)C)C(=O)NC(C)(C)C(=O)NC(C)(C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)Cc3ccccc3
InChI
1S/C92H150N22O25/c1-47(2)43-58(72(127)108-92(24,25)84(139)113-41-29-33-59(113)73(128)103-65(48(3)4)75(130)111-90(20,21)82(137)112-89(18,19)80(135)102-56(37-40-64(120)121)70(125)101-55(35-38-61(93)117)69(124)98-54(46-115)44-53-31-27-26-28-32-53)99-63(119)45-95-77(132)85(10,11)110-76(131)66(49(5)6)104-81(136)88(16,17)107-71(126)57(36-39-62(94)118)100-67(122)50(7)96-78(133)86(12,13)106-68(123)51(8)97-79(134)87(14,15)109-74(129)60-34-30-42-114(60)83(138)91(22,23)105-52(9)116/h26-28,31-32,47-51,54-60,65-66,115H,29-30,33-46H2,1-25H3,(H2,93,117)(H2,94,118)(H,95,132)(H,96,133)(H,97,134)(H,98,124)(H,99,119)(H,100,122)(H,101,125)(H,102,135)(H,103,128)(H,104,136)(H,105,116)(H,106,123)(H,107,126)(H,108,127)(H,109,129)(H,110,131)(H,111,130)(H,112,137)(H,120,121)/t50-,51-,54+,55-,56-,57-,58-,59-,60-,65-,66-/m0/s1
Nemzetközi kémiai azonosító kulcs
LGHSQOCGTJHDIL-SLKIUSOBSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
- to examine the effect of glutamate transporters on the Na+/K+-ATPase dependent extracellular K+ transient during neuronal activity
- to investigate the formation of anion-permeable channels in planar lipid bilayers by magainin I obtained from Xenopus skin
- to study the permeability of root apical meristem and epidermis and cellulase induced resistance to Alamethicin in Arabidopsis thaliana
- to study the cytotoxic effects of the phycotoxin okadaic acid and mycotoxins on human intestinal (HT-29) and neuroblastoma (SH-SY5Y) cell lines
- to analyze the structural variations and biological activity of peptaibols obtained from the Longibrachiatum clade belonging to the genus of filamentous fungi Trichoderma
Biokémiai/fiziológiai hatások
Minőség
Szekvencia
Figyelmeztetés
Danger
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Acute Tox. 3 Oral
Tárolási osztály kódja
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással