Fontos dokumentumok
909459
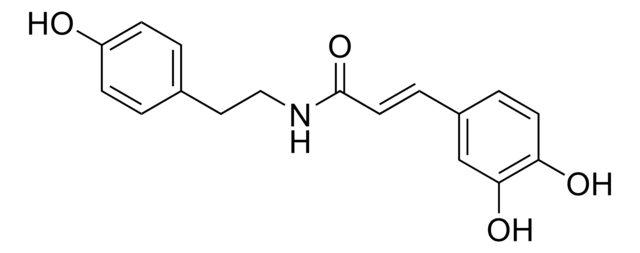
Fluorinated VHL Spy Molecule 4
≥98%
Szinonimák:
(2S,4R)-N-(4-bromobenzyl)-4-hydroxy-1-(3,3,3-trifluoropropanoyl)pyrrolidine-2-carboxamide
About This Item
Javasolt termékek
ligand
VH032 derivative
Teszt
≥98%
form
powder
SMILES string
O=C([C@@H]1C[C@@H](O)CN1C(CC(F)(F)F)=O)NCC2=CC=C(Br)C=C2
Alkalmazás
The E3 ligase VHL is of growing interest for targeted protein degradation, and these spy molecules will facilitate the identification of novel VHL ligands and VHL-based degraders.
Egyéb megjegyzések
Structure-guided design and optimization of small molecules targeting the protein-protein interaction between the von Hippel-Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) alpha subunit with in vitro nanomolar affinities
kapcsolódó termék
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Sajnos jelenleg COA nem áll rendelkezésre ehhez a termékhez online.
Ha segítségre van szüksége, lépjen velünk kapcsolatba Vevőszolgálat
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Related Content
The Ciulli group are broadly interested with understanding and exploiting the ligandability of protein-protein interactions (PPIs) and protein surfaces within complex biological systems using chemical and structural cell biology approaches. Current research efforts are directed towards targeting PPIs molecular recognition within protein families of biological and medical relevance within the Ubiquitin and Chromatin systems by developing small molecules that disrupt PPIs and that induce targeted protein degradation (PROTAC®) - as tools to probe biology and leads for drug discovery.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással