Key Documents
569747
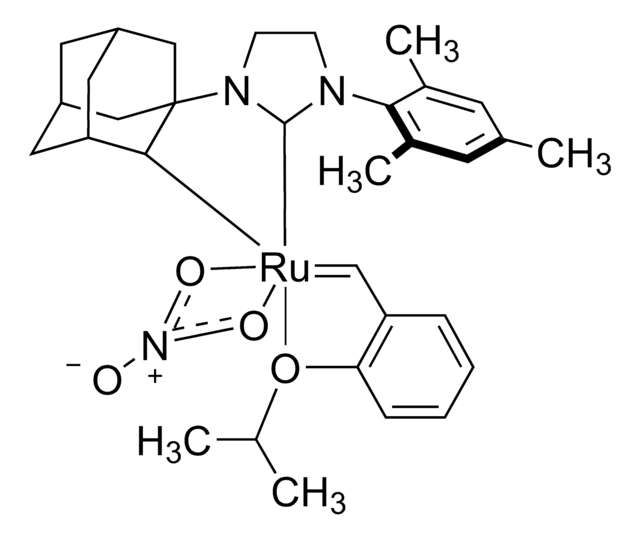
Grubbs Catalyst® M204
Umicore
Synonim(y):
(1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium, Benzylidene[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(tricyclohexylphosphine)ruthenium, Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene](benzylidene)(tricyclohexylphosphine)ruthenium(II), Grubbs Catalyst® 2nd Generation, Grubbs Catalyst® M2a (C848)
About This Item
Polecane produkty
Poziom jakości
Postać
solid
przydatność reakcji
core: ruthenium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
mp
143.5-148.5 °C
temp. przechowywania
2-8°C
ciąg SMILES
CC1=CC(C)=CC(C)=C1N2CCN(C3=C(C)C=C(C)C=C3C)C2=[Ru](Cl)(Cl)=CC4=CC=CC=C4.P(C5CCCCC5)(C6CCCCC6)C7CCCCC7
InChI
1S/C21H26N2.C18H33P.C7H6.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-7-5-3-2-4-6-7;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1-6H;2*1H;/q;;;;;+2/p-2
Klucz InChI
FCDPQMAOJARMTG-UHFFFAOYSA-L
Zastosowanie
It can also be used as a catalyst:
- To synthesize coumarins from phenolic compounds via RCM.
- To cleave secondary (E)-allyl vic-diols to aldehydes.
Learn more about our metathesis catalysts
Inne uwagi
Informacje prawne
Product License
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
produkt powiązany
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Flam. Sol. 2
Kod klasy składowania
4.1B - Flammable solid hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Learn tips and tricks for how to properly use inhibitors including how to select the right inhibitor and how to plan experiments with inhibitors.
Protokoły
TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
Środek powierzchniowo czynny TPGS-750-M umożliwia różne reakcje w wodzie w temperaturze pokojowej, zwiększając wydajność i wszechstronność syntezy.
Powiązane treści
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej










![2,6-Diisopropylphenylimido-neophylidene[(S)-(−)-BIPHEN]molybdenum(VI) ringclosing metathesis catalyst, ≥95.0% (C)](/deepweb/assets/sigmaaldrich/product/structures/312/745/96ea840b-77a7-427a-9db5-fa08b3ffd45e/640/96ea840b-77a7-427a-9db5-fa08b3ffd45e.png)
![Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene][[5-[(dimethylamino)sulfonyl]-2-(1-methylethoxy-O)phenyl]methylene-C]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/179/573/f48a2a1e-cf09-4151-8b78-2bab614efd5c/640/f48a2a1e-cf09-4151-8b78-2bab614efd5c.png)
