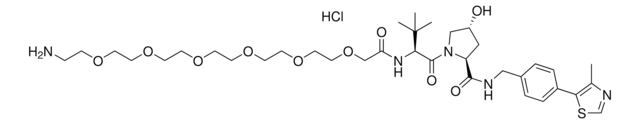
905380
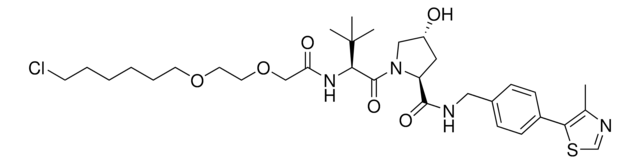
(S,R,S)-AHPC-C6-PEG3-butyl chloride
≥95%
別名:
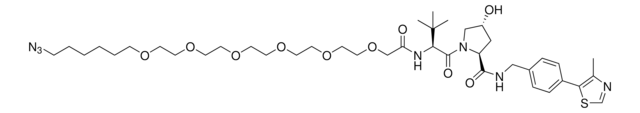
(2S,4R)-1-((S)-2-(tert-Butyl)-22-chloro-4-oxo-10,13,16-trioxa-3-azadocosanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide, (S,R,S)-AHPC-6-2-2-6-Cl, Crosslinker−E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
おすすめの製品
ligand
VH032
アッセイ
≥95%
形状
solid
反応適合性
reactivity: sulfuryl reactive
reagent type: ligand-linker conjugate
官能基
alkyl halide
保管温度
2-8°C
SMILES記法
ClCCCCCCOCCOCCOCCCCCC(N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C)=O
アプリケーション
その他情報
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
法的情報
関連製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
905380-BULK:
905380-VAR:
905380-50MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
資料
タンパク質分解誘導化合物ビルディングブロックは、標的リガンドと共有結合するペンダント基を持つクロスリンカー-E3リガンド複合体です。
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)