Fontos dokumentumok
SML2916
AAL-R
≥98% (HPLC)
Szinonimák:
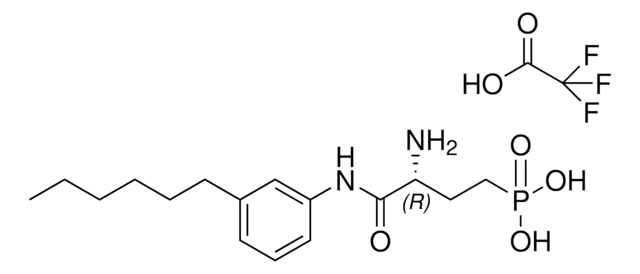
(R)-2-Amino-2-methyl-4-[4-(heptyloxy)phenyl]butan-1-ol, (R)-2-Amino-4-(4-(heptyloxy)phenyl)-2-methylbutan-1-ol, (R)-2-Amino-4-(4-heptyloxyphenyl)-2-methylbutanol, AAL(R)
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥98% (HPLC)
form
powder
szín
white to beige
oldhatóság
DMSO: 2 mg/mL, clear
tárolási hőmérséklet
2-8°C
SMILES string
C[C@](N)(CO)CCC1=CC=C(C=C1)OCCCCCCC
Biokémiai/fiziológiai hatások
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással