Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Fontos dokumentumok
P6635
Phosphorylase b from rabbit muscle
lyophilized powder, ≥20 units/mg protein, 2× crystallization
Szinonimák:
α-Glucan Phosphorylase, 1,4-α-D-Glucan:orthophosphate α-D-glucosyltransferase, Glycogen Phosphorylase
Méret kiválasztása
41 500,00 Ft
Méret kiválasztása
About This Item
41 500,00 Ft
Javasolt termékek
biológiai forrás
rabbit muscle
Minőségi szint
Forma
lyophilized powder
specifikus aktivitás
≥20 units/mg protein
molekulatömeg
97,200 Da by calculation
tisztítva
2× crystallization
tárolási körülmény
(Keep container tightly closed in a dry and well-ventilated place)
technika/technikák
mass spectrometry (MS): suitable
szennyeződések
~0.01 μmol/mg protein 5′-AMP (This low level will not interfere with phosphorylase and phosphorylase kinase assays.)
UniProt elérési szám
idegen aktivitás
phosphoglucomutase ≤1.0%
phosphorylase a ≤10%
phosphorylase kinase ≤0.5%
phosphorylase phosphatase, debrancher enzyme, AMPase and ATPase ≤0.1%
tárolási hőmérséklet
−20°C
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
- in the calibration of Sepharose C1-6B columns while studying the molecular weight of methylamine dehydrogenase subunits[3]
- in ion mobility-mass spectrometry studies of phosphorylase B ions that have been generated with supercharging reagents, in the charge-reducing buffer[4]
- for the preparation of p32 labeled phosphorylase A using phosphorylase kinase and [32P]ATP[5]
- in phosphorylase phosphatase assay[6]
- in enzyme assay as a positive control to ensure the reaction system for the activity determination was adopted[7]
Biokémiai/fiziológiai hatások
Kiszerelés
Egység definíció
Fizikai forma
antitest
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
Has the amino acid sequence of phosphorylase from rabbit muscle been published?
1 answer-
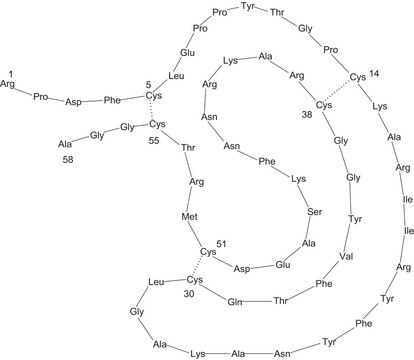
The sequence has been published. The amino acid composition is:Asp5l, Asn45, Thr35, Ser29, Glu64, Gln31, Pro36, Gly48, Ala63, Cys9, Val62, Met2l, Ile49, Leu79, Tyr36, Phe38, Trp12, Lys48, HiS22, and Arg63. Reference for the sequence and amino acid composition: K. Titani et al., Proc. Nat. Acad. Sci., USA, 74, 4762-4766 (1977) (see attached pdf).Product P1261 is phosphorylase a, which is the tetrameric form. Products P4649 and P6635 are phosphorylase b, the dimeric form. The sequence information of the subunit would be the same for both forms.
Helpful?
-
Active Filters
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással