Fontos dokumentumok
C8868
Cholesterol Oxidase from microorganisms
lyophilized powder, ≥50 units/mg protein, recombinant, expressed in E. coli
Szinonimák:
Cholesterol: oxygen oxidoreductase
About This Item
Javasolt termékek
rekombináns
expressed in E. coli
Minőségi szint
Forma
lyophilized powder
specifikus aktivitás
≥50 units/mg protein
molekulatömeg
64 kDa
összetétel
Protein, ≥15% biuret
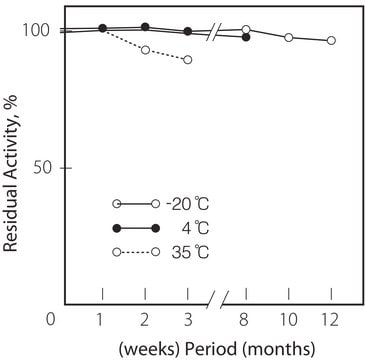
tárolási hőmérséklet
−20°C
Alkalmazás
Biokémiai/fiziológiai hatások
Fizikai tulajdonságok
Egység definíció
Fizikai forma
Figyelmeztetés
Danger
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Resp. Sens. 1
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Protocols
Enzymatic activity assay for cholesterol oxidase, excluding specific product numbers, providing guidelines for accurate cholesterol oxidase assays.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással