AB9234
Anti-Amyloid Oligomer Antibody, αβ, oligomeric
serum, Chemicon®
Szinonimák:
Anti-oligomer
About This Item
Javasolt termékek
biológiai forrás
rabbit
Minőségi szint
antitest forma
serum
antitest terméktípus
primary antibodies
klón
polyclonal
faj reaktivitás
rat, eukaryotes, mouse
faj reaktivitás (homológia által előrejelzett)
human
gyártó/kereskedő neve
Chemicon®
technika/technikák
ELISA: suitable
immunofluorescence: suitable
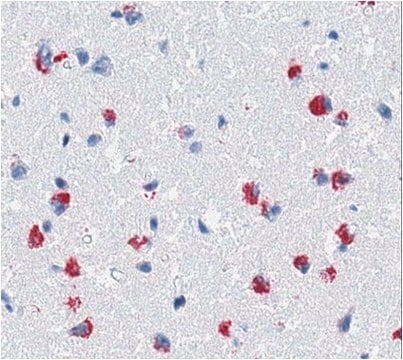
immunohistochemistry (formalin-fixed, paraffin-embedded sections): suitable
immunoprecipitation (IP): suitable
western blot: suitable
NCBI elérési szám
UniProt elérési szám
kiszállítva
dry ice
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... APP(351)
Általános leírás
Egyediség
Immunogen
Alkalmazás
A 1:1,000-1:10,000 concentration was used on a previous lot.
Immunoprecipitation:
A 1:1,000 concentration was used on a previous lot. Suggested cell lysis buffer is RIPA. Suggested capture agent is magnetic beads (Dynabeads). Known co-precipitatiing polypeptide: Amyloid beta, alpha synuclein oligomers.
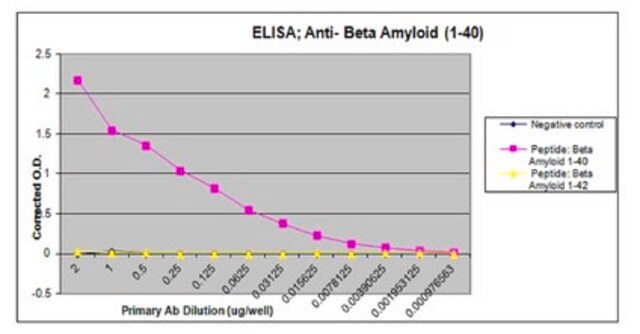
ELISA (direct):
A previous lot of this antibody was used in ELISA.
Optimal working dilutions must be determined by the end user.
Neuroscience
Neurodegenerative Diseases
Minőség
Western Blotting Analysis:
1:500 dilution of this antibody detected AMYLOID OLIGOMER on 10 μg of mouse brain lysates.
Fizikai forma
Tárolás és stabilitás
Analízis megjegyzés
Brain
Egyéb megjegyzések
Jogi információk
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
Tárolási osztály kódja
12 - Non Combustible Liquids
WGK
WGK 1
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással