AB2287
Anti-Amyloid Fibrils LOC Antibody
serum, Chemicon®
Szinonimák:
Amyloid Fibrils, Amyloid Fibrils LOC
About This Item
Javasolt termékek
biológiai forrás
rabbit
Minőségi szint
antitest forma
serum
antitest terméktípus
primary antibodies
klón
polyclonal
faj reaktivitás
human
faj reaktivitás (homológia által előrejelzett)
mouse, rat
gyártó/kereskedő neve
Chemicon®
technika/technikák
ELISA: suitable
dot blot: suitable
immunocytochemistry: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
izotípus
IgG
NCBI elérési szám
UniProt elérési szám
kiszállítva
wet ice
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... APP(351)
mouse ... App(11820)
Általános leírás
Egyediség
Immunogen
Alkalmazás
Minőség
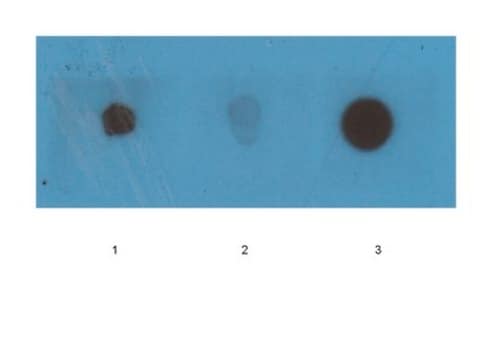
Dot Blot Analysis: 1:1,000 dilution of this antibody detected Amyloid fibrils in fibrils and monomers but not in prefibril oligos. A 1:5,000 dilution, as cited in Glabe C., et al. (2007) Mol Neurodegener 2, 18 shows that the binding with monomers is likely non-specific, and is a possible result of high primary antibody concentration.
Tárolás és stabilitás
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance. After thawing, store at 4°C in 0.02% sodium azide.
Jogi információk
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
Tárolási osztály kódja
10 - Combustible liquids
WGK
WGK 1
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással