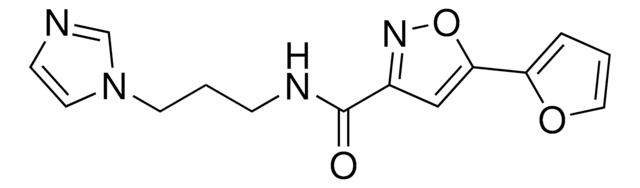
681669
IWR-1-endo
≥95% (HPLC), solid, Wnt antagonist, Calbiochem®
Szinonimák:
Wnt Antagonist I, IWR-1-endo, Inhibitor of Wnt Response-1, Wnt Pathway Inhibitor I, TNKS1/2 Inhibitor II, Tankyrase1/2 Inhibitor II, Wnt Pathway Inhibitor I, Inhibitor of Wnt Response-1, Tankyrase1/2 Inhibitor II, TNKS1/2 Inhibitor II
About This Item
Javasolt termékek
product name
Wnt Antagonist I, IWR-1-endo, Wnt Antagonist I, IWR-1-endo, is a cell-permeable inhibitor of TNKS1/PARP5a (IC₅₀ = 131 nM) & TNKS2/PARP5b (IC₅₀= 56 nM). Suppress Wnt-stimulated transcription activity (IC₅₀ = 180 nM).
Minőségi szint
Teszt
≥95% (HPLC)
form
solid
gyártó/kereskedő neve
Calbiochem®
tárolási körülmény
OK to freeze
protect from light
szín
off-white
oldhatóság
DMSO: 10 mg/mL
kiszállítva
ambient
tárolási hőmérséklet
2-8°C
Általános leírás
Kiszerelés
Figyelmeztetés
Feloldás
Egyéb megjegyzések
Huang, S.M., et al. 2009. Nature461, 614.
Jogi információk
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással