203290
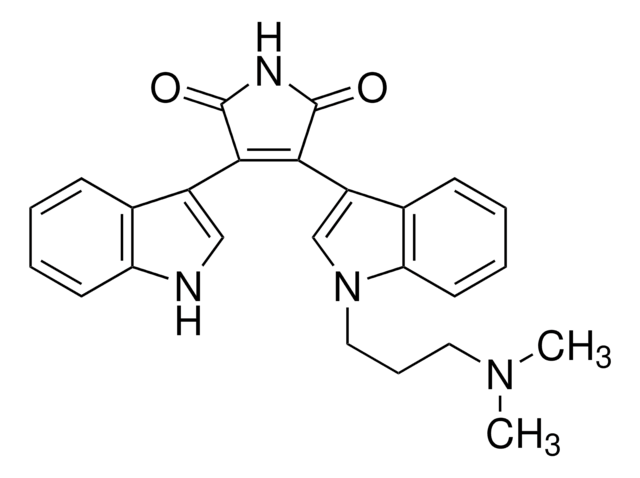
Bisindolylmaleimide I
A highly selective, cell-permeable, and reversible protein kinase C (PKC) inhibitor (IC₅₀ = 10 nM) that is structurally similar to staurosporine.
Szinonimák:
Bisindolylmaleimide I, 2-[1-(3-Dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide, Gö 6850, GF 109203X
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥95% (HPLC)
form
solid
gyártó/kereskedő neve
Calbiochem®
tárolási körülmény
OK to freeze
protect from light
szín
deep orange
oldhatóság
DMSO: 10 mg/mL
kiszállítva
ambient
tárolási hőmérséklet
2-8°C
InChI
1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31)
Nemzetközi kémiai azonosító kulcs
QMGUOJYZJKLOLH-UHFFFAOYSA-N
Általános leírás
Biokémiai/fiziológiai hatások
PKC
Figyelmeztetés
Elkészítési megjegyzés
Feloldás
Egyéb megjegyzések
Ku, W.-C., et al. 1997. Biochem. Biophys. Res. Commun. 241, 730.
Gekeler, V., et al. 1996. Br. J. Cancer 74, 897.
Kiss, Z., et al. 1995. Biochim. Biophys. Acta 1265, 93.
Toullec, D., et al. 1991. J. Biol. Chem. 266, 15771.
Jogi információk
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Cikkek
Learn tips and tricks for how to properly use inhibitors including how to select the right inhibitor and how to plan experiments with inhibitors.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással