Fontos dokumentumok
718742
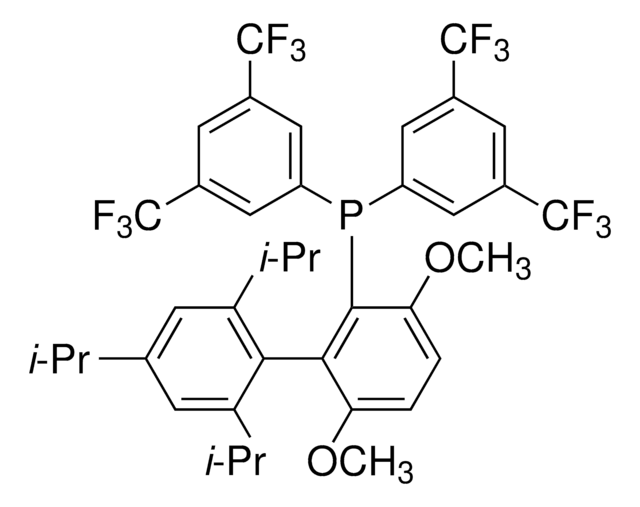
BrettPhos
98%
Szinonimák:
2-(Dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl
About This Item
Javasolt termékek
Minőségi szint
Teszt
98%
Forma
solid
reakcióalkalmasság
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Fluorinations
környezetbarátabb alternatív termék pontszám
old score: 8
new score: 1
Find out more about DOZN™ Scoring
környezetbarátabb alternatív termék tulajdonságai
Waste Prevention
Atom Economy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-195 °C
funkcionális csoport
phosphine
környezetbarátabb alternatív kategória
SMILES string
COc1c(P(C2CCCCC2)C3CCCCC3)c(c4c(C(C)C)cc(C(C)C)cc4C(C)C)c(OC)cc1
InChI
1S/C35H53O2P/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28/h19-25,27-28H,9-18H2,1-8H3
Nemzetközi kémiai azonosító kulcs
WDVGNXKCFBOKDF-UHFFFAOYSA-N
Általános leírás
Alkalmazás
It can be used in:
- palladium-catalyzed trifluoromethylation of aryl chlorides
- Buchwald-Hartwig amination
- synthesis of 4-aryl and alkyl substituted, N6-alkylated pyridazine-3,6-diamines via a Buchwald protocol
Tulajdonságok és előnyök
- White crystalline solid
- Air- and moisture-stable
- Thermally stable
- Highly efficient
- Wide functional group tolerance
- Excellent selectivity and conversion
Tárolási osztály kódja
11 - Combustible Solids
WGK
nwg
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Related Content
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással











![[2-(Dicyclohexylphosphino)-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl]gold(I) bis(trifluoromethanesulfonyl)imide](/deepweb/assets/sigmaaldrich/product/structures/361/949/e30e9505-889a-4ffd-9c57-f66a0a20b299/640/e30e9505-889a-4ffd-9c57-f66a0a20b299.png)


