Fontos dokumentumok
422177
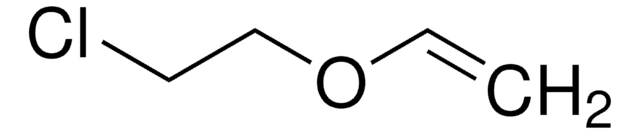
Ethyl vinyl ether
contains 0.1% KOH as stabilizer, 99%
Szinonimák:
Ethoxyethylene
About This Item
Javasolt termékek
Minőségi szint
Teszt
99%
Forma
liquid
tartalmaz
0.1% KOH as stabilizer
0.1% potassium hydroxide as stabilizer
törésmutató
n20/D 1.376 (lit.)
bp
33 °C (lit.)
mp
−116 °C (lit.)
sűrűség
0.753 g/mL at 25 °C (lit.)
tárolási hőmérséklet
2-8°C
SMILES string
CCOC=C
InChI
1S/C4H8O/c1-3-5-4-2/h3H,1,4H2,2H3
Nemzetközi kémiai azonosító kulcs
FJKIXWOMBXYWOQ-UHFFFAOYSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
Ethyl vinyl ether can be used as:
- A monomer to synthesize amphiphilic block copolymers to fabricate protein-repelling polymersomes to produce spherical nanoparticles. These nanoparticles can be used as drug carriers.
- A precursor to synthesize polymer electrolytes and cathode materials for solid-state lithium-ion batteries via UV photopolymerizations.
- A monomer to synthesize poly(vinyl ether)s with controlled molecular weight and narrow dispersity via photoinduced free radical-promoted cationic reversible addition–fragmentation chain transfer (RAFT) polymerization. These polymers are used to fabricate 3D objects with different thicknesses by employing stereolithography-based 3D printing.
- H-bonded Reusable Template Assisted para-Selective Ketonisation: This study discusses the use of ethyl vinyl ether in catalytic processes involving palladium and hexafluoroisopropanol to achieve para-selectivity in ketonisation, relevant for synthesizing complex organic compounds used in pharmaceuticals and materials science (A Maji, A Dahiya, G Lu, T Bhattacharya, 2018).
- Mechanistic Insight into Photocontrolled Cationic Polymerization: Explores the photocontrolled polymerization of ethyl vinyl ether, providing valuable knowledge for the development of light-responsive materials, which could have applications in drug delivery and smart material systems (Q Michaudel, T Chauviré, V Kottisch, 2017).
Figyelmeztetés
Danger
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Aquatic Chronic 3 - Flam. Liq. 2 - STOT SE 3
Célzott szervek
Central nervous system
Tárolási osztály kódja
3 - Flammable liquids
WGK
WGK 1
Lobbanási pont (F)
-49.0 °F
Lobbanási pont (C)
-45 °C
Egyéni védőeszköz
Eyeshields, Faceshields, Gloves
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással