920843
(S,R,S)-VL285 Phenol-PEG1-piperazine hydrochloride
Synonim(y):
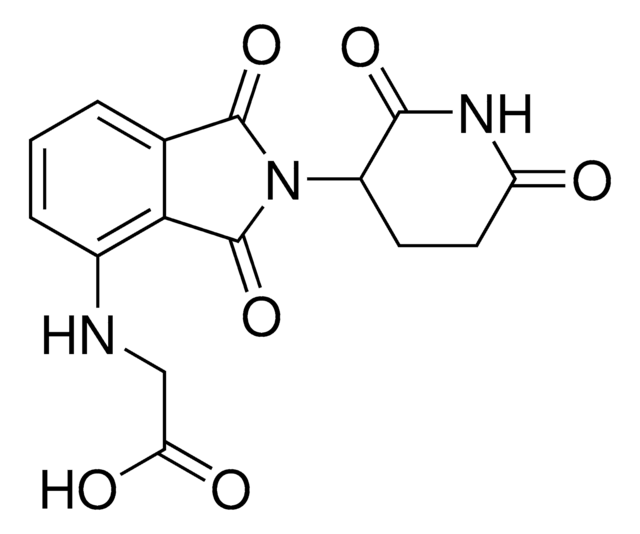
(2S,4R)-4-Hydroxy-N-{[4-(4-methyl-1,3-thiazol-5-yl)-2-{2-[2-(piperazin-1-yl)ethoxy]ethoxy}phenyl]methyl}-1-[(2S)-3-methyl-2-(1-oxo-2,3-dihydro-1H-isoindol-2-yl)butanoyl]pyrrolidine-2-carboxamide hydrochloride, Crosslinker−E3 Ligase ligand conjugate, VHL protein degrader building block for PROTAC® research
About This Item
Polecane produkty
ligand
VL285 phenol
Poziom jakości
Postać
solid
przydatność reakcji
reagent type: ligand-linker conjugate
temp. przechowywania
2-8°C
ciąg SMILES
O=C([C@@H]1C[C@@H](O)CN1C([C@H](C(C)C)N2CC(C=CC=C3)=C3C2=O)=O)NCC4=CC=C(C5=C(C)N=CS5)C=C4OCCOCCN6CCNCC6.Cl
Zastosowanie
Inne uwagi
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
Informacje prawne
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej