Kluczowe dokumenty
919489
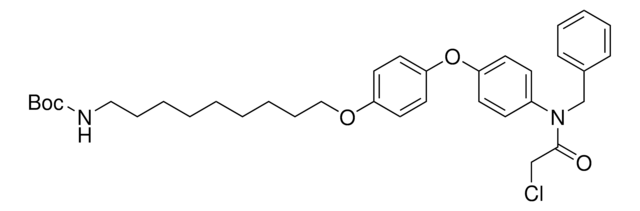
CCW16-C6-PEG3-butyl-BocNH
Synonim(y):
(6-(2-(2-((6-(4-(4-(N-benzylo-2-chloroacetamido)fenoksy)fenoksy)heksylo)oksy)etoksy)heksy)heksylo)karbaminian) tert-butylu, Blok konstrukcyjny celujący w RNF4, Blok konstrukcyjny degradera białek dla badań 11779, Koniugat ligandu Crosslinker-E3 Ligase, Szablon do syntezy ukierunkowanego degradera białek
About This Item
Polecane produkty
ligand
CCW16
Poziom jakości
Formularz
viscous liquid
przydatność reakcji
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
grupa funkcyjna
amine
temp. przechowywania
2-8°C
ciąg SMILES
O=C(CCl)N(CC1=CC=CC=C1)C2=CC=C(C=C2)OC3=CC=C(OCCCCCCOCCOCCOCCCCCCNC(OC(C)(C)C)=O)C=C3
InChI
1S/C42H59ClN2O8/c1-42(2,3)53-41(47)44-25-11-4-5-12-26-48-29-31-50-32-30-49-27-13-6-7-14-28-51-37-21-23-39(24-22-37)52-38-19-17-36(18-20-38)45(40(46)33-43)34-35-15-9-8-10-16-35/h8-10,15-24H,4-7,11-14,25-34H2,1-3H3,(H,44,47)
Klucz InChI
MBDBAPHHKCSIAT-UHFFFAOYSA-N
Powiązane kategorie
Zastosowanie
Inne uwagi
Portal: Budowa 11779 degraderów do ukierunkowanej degradacji białek
Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: Nowe podejścia do degradacji białek
Ukierunkowana degradacja białek: od biologii chemicznej do odkrywania leków
Informacje prawne
produkt powiązany
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej


![Chloro[tris(para-trifluoromethylphenyl)phosphine]gold(I) 99%](/deepweb/assets/sigmaaldrich/product/structures/250/453/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e/640/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e.png)