Fontos dokumentumok
M7571
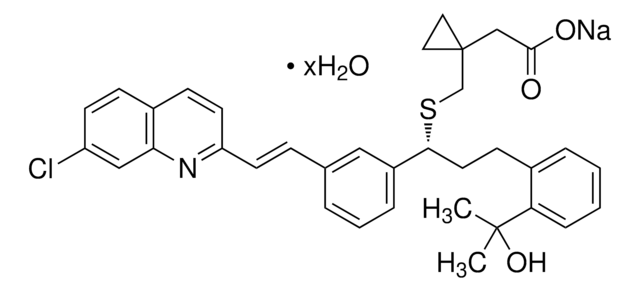
MK-571 sodium salt hydrate
≥95% (HPLC), powder, leukotriene D4 antagonist
Szinonimák:
5-(3-(2-(7-Chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid sodium salt hydrate, L-660711
About This Item
Javasolt termékek
product name
MK-571 sodium salt hydrate, ≥95% (HPLC)
Minőségi szint
Teszt
≥95% (HPLC)
form
powder
tárolási körülmény
desiccated
szín
white to beige
oldhatóság
H2O: 15 mg/mL, clear
kezdeményező
Merck & Co., Inc., Kenilworth, NJ, U.S.
kiszállítva
wet ice
tárolási hőmérséklet
−20°C
SMILES string
O.[Na+].CN(C)C(=O)CCSC(SCCC([O-])=O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1
InChI
1S/C26H27ClN2O3S2.Na.H2O/c1-29(2)24(30)12-14-33-26(34-15-13-25(31)32)20-5-3-4-18(16-20)6-10-22-11-8-19-7-9-21(27)17-23(19)28-22;;/h3-11,16-17,26H,12-15H2,1-2H3,(H,31,32);;1H2/q;+1;/p-1/b10-6+;;
Nemzetközi kémiai azonosító kulcs
MSHRPLRGSQECLY-DOLBFOAYSA-M
Alkalmazás
- as an efflux inhibitor for monitoring multidrug resistance protein (MRP)-function and to avoid redundancy of other transporters
- to assess its effect on cell proliferation and 2D-migration in vitro in various cell lines of glioblastoma multiforme (GBM)
- as multidrug resistance (MDR) transporter inhibitor to study its effects in ovarian cancer cells
- as specific inhibitors of ABCC1/2 to investigate transport, toxicity, flow cytometry and arsenic efflux
Biokémiai/fiziológiai hatások
Tulajdonságok és előnyök
Figyelmeztetés
Warning
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Célzott szervek
Respiratory system
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
dust mask type N95 (US), Eyeshields, Gloves
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Cikkek
We offer many products related to leukotriene receptors for your research needs.
Discover Bioactive Small Molecules for Lipid Signaling Research
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással