Fontos dokumentumok
H4645
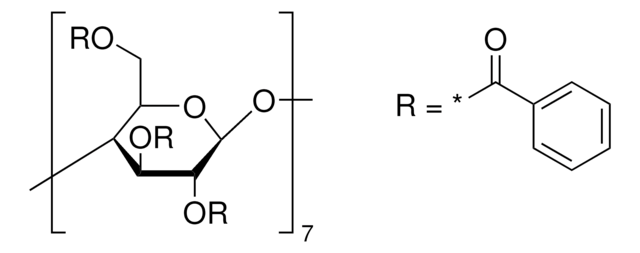
Heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin
≥90%
Szinonimák:
2,3,6-Tri-O-methyl-β-cyclodextrin, Trimethyl-β-cyclodextrin
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥90%
Forma
powder
technika/technikák
electrophoresis: suitable
mp
170-178 °C (lit.)
tárolási hőmérséklet
2-8°C
SMILES string
COC[C@H]1O[C@@H]2O[C@@H]3[C@@H](COC)O[C@H](O[C@@H]4[C@@H](COC)O[C@H](O[C@@H]5[C@@H](COC)O[C@H](O[C@@H]6[C@@H](COC)O[C@H](O[C@@H]7[C@@H](COC)O[C@H](O[C@@H]8[C@@H](COC)O[C@H](O[C@H]1[C@H](OC)[C@H]2OC)[C@H](OC)[C@H]8OC)[C@H](OC)[C@H]7OC)[C@H](OC)[C@H]6OC)[C@H](OC)[C@H]5OC)[C@H](OC)[C@H]4OC)[C@H](OC)[C@H]3OC
InChI
1S/C63H112O35/c1-64-22-29-36-43(71-8)50(78-15)57(85-29)93-37-30(23-65-2)87-59(52(80-17)44(37)72-9)95-39-32(25-67-4)89-61(54(82-19)46(39)74-11)97-41-34(27-69-6)91-63(56(84-21)48(41)76-13)98-42-35(28-70-7)90-62(55(83-20)49(42)77-14)96-40-33(26-68-5)88-60(53(81-18)47(40)75-12)94-38-31(24-66-3)86-58(92-36)51(79-16)45(38)73-10/h29-63H,22-28H2,1-21H3/t29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43+,44+,45+,46+,47+,48+,49+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-/m1/s1
Nemzetközi kémiai azonosító kulcs
DSDAICPXUXPBCC-MWDJDSKUSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
- To investigate the crystal structure of its complexes with m-iodophenol, 4-biphenylacetic acid and (R)- and (S)-flurbiprofen by X-ray analysis.
- To study the candidature of its complex with vitamin A for potential application as a drug delivery system for ophthalmic applications by high sensitivity fluorescence spectrometry and high pressure liquid chromatography (HPLC) techniques.
- In the determination of the analyte composition in commercial samples by HPLC coupled to mass spectrometry (MS).
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, type N95 (US)
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással