Fontos dokumentumok
C7974
Monoclonal Anti-Collagen, Type X antibody produced in mouse
clone COL-10, ascites fluid
Szinonimák:
Anti-Col10, Anti-Col10a-1
About This Item
Javasolt termékek
biológiai forrás
mouse
Minőségi szint
konjugátum
unconjugated
antitest forma
ascites fluid
antitest terméktípus
primary antibodies
klón
COL-10, monoclonal
molekulatömeg
antigen 60 kDa (in denatured-reduced preparations)
tartalmaz
15 mM sodium azide
faj reaktivitás
deer, human, pig
technika/technikák
dot blot: suitable
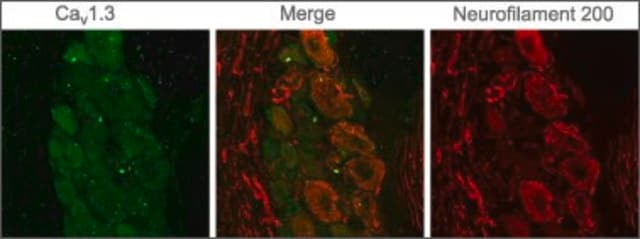
immunocytochemistry: 1:1,000 using HT 1080 human fibrosarcoma cells
western blot: suitable
izotípus
IgM
UniProt elérési szám
kiszállítva
dry ice
tárolási hőmérséklet
−20°C
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... COL10A1(1300)
Related Categories
Általános leírás
Immunogén
Alkalmazás
- enzyme linked immunosorbent assay (ELISA)
- dot-blot
- immunoblotting and
- immunohistochemistry
Biokémiai/fiziológiai hatások
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
javasolt
Tárolási osztály kódja
10 - Combustible liquids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással