Fontos dokumentumok
C1999
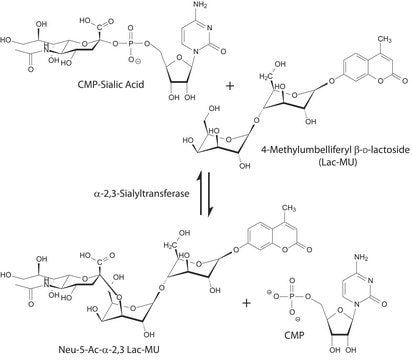
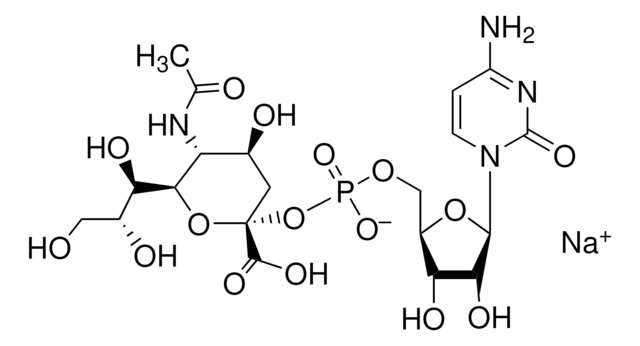
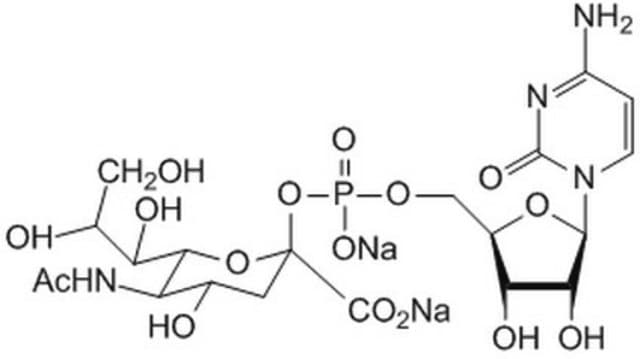
CMP-Sialic Acid Synthetase from Neisseria meningitidis group B
recombinant, expressed in E. coli BL21, ≥10 units/mg protein
Szinonimák:
CTP: N-Acylneuraminate cytidylyltransferase
About This Item
Javasolt termékek
rekombináns
expressed in E. coli BL21
Minőségi szint
Forma
lyophilized solid
specifikus aktivitás
≥10 units/mg protein
molekulatömeg
26.0 kDa
kiszállítva
dry ice
tárolási hőmérséklet
−20°C
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Related Categories
Általános leírás
Alkalmazás
Biokémiai/fiziológiai hatások
Egység definíció
Fizikai forma
Analízis megjegyzés
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Cikkek
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Enzymatic glycosyltransferase specificity challenges the one enzyme-one linkage concept.
Understand sialic acid structure, function, signaling, and modifications. Easily find products for sialic acid research.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással