Fontos dokumentumok
383120
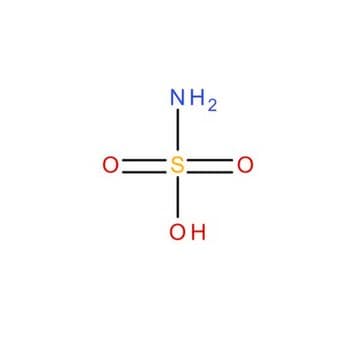
Sulfamic acid
ACS reagent, 99.3%
Szinonimák:
Amidosulfonic acid
About This Item
Javasolt termékek
grade
ACS reagent
Minőségi szint
Teszt
99.3%
99.3-100.3% dry basis (ACS specification)
Forma
crystals
technika/technikák
titration: suitable
szennyeződések
≤0.01% insolubles
izzítási maradék
≤0.01%
mp
215-225 °C (dec.) (lit.)
oldhatóság
water: 213 g/L at 20 °C
sűrűség
2.151 g/cm3 at 25 °C
anion nyomok
chloride (Cl-): ≤0.001%
sulfate (SO42-): ≤0.05%
kation nyomok
Fe: ≤5 ppm
heavy metals (as Pb): ≤0.001%
SMILES string
NS(O)(=O)=O
InChI
1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)
Nemzetközi kémiai azonosító kulcs
IIACRCGMVDHOTQ-UHFFFAOYSA-N
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Related Categories
Általános leírás
Alkalmazás
- As catalyst in the synthesis of aryl-14H-dibenzo[a.j]xanthenes.
- As green catalyst for the preparation of amide from ketoxime.
- As ammonia equivalent in the regioselective synthesis of primary allylic amines, via allylic substitution reactions.
- Synthesis of polysubstituted quinolones.
- As a titrant in the determination of the burette injection volume and chemical calibration factor.
- To neutralize excess nitrous acid in the colorimetric paracetamol assay by modified Glynn and Kendal colorimetric method.
- To prevent endogenous mercury (Hg) loss during the urine Hg measurement by inductively coupled plasma mass spectrometry (ICP-MS) method.
- As an acid catalyst and a hypochlorite scavenger in the chlorite oxidation of dialdehyde cellulose (DAC).
- As a heterogeneous catalyst in the synthesis of polyhydroquinoline derivatives by Hantzsch condensation reaction.
- As catalyst in the degradation of bamboo fiber to 5-hydroxymethylfurfural (HMF).
- As an acid reagent in the determination of silicates in water samples based on centrifugal microfluidics.
- As catalyst in the synthesis of deazaoxaflavin at room temperature.
Figyelmeztetés
Warning
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Tárolási osztály kódja
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással