Fontos dokumentumok
936596
1H-Isoindole-1,3(2H)-dione, 4-[(4-aminobutyl)amino]-2-(2,6-dioxo-3-piperidinyl)-, hydrochloride
≥95%
Szinonimák:
4-((4-aminobutyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥95%
Forma
powder or crystals
szín
light yellow to yellow
tárolási hőmérséklet
2-8°C
SMILES string
Cl.O=C1NC(=O)C(N2C(=O)C=3C=CC=C(NCCCCN)C3C2=O)CC1
InChI
1S/C17H20N4O4.ClH/c18-8-1-2-9-19-11-5-3-4-10-14(11)17(25)21(16(10)24)12-6-7-13(22)20-15(12)23;/h3-5,12,19H,1-2,6-9,18H2,(H,20,22,23);1H
Alkalmazás
Technology Spotlight:
Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Egyéb megjegyzések
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Jogi információk
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Válasszon a legfrissebb verziók közül:
Analitikai tanúsítványok (COA)
Nem találja a megfelelő verziót?
Ha egy adott verzióra van szüksége, a tétel- vagy cikkszám alapján rákereshet egy adott tanúsítványra.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással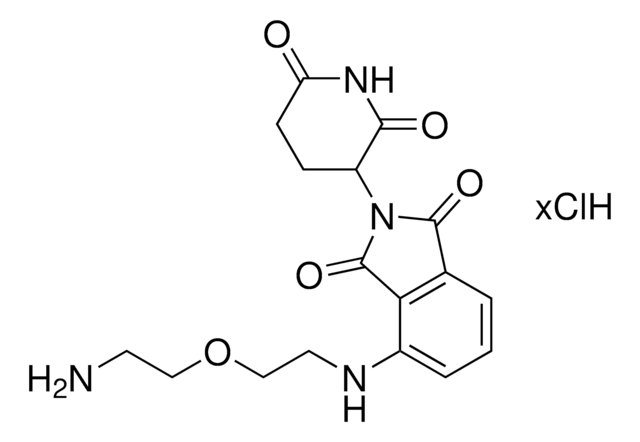
![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)

![[4,4′-Dimethyl-2,2′-bipyridine]nickel(II) dichloride hydrate ≥95%](/deepweb/assets/sigmaaldrich/product/structures/714/807/6f838a65-cb7b-400f-bdb3-3e207bb2ddc4/640/6f838a65-cb7b-400f-bdb3-3e207bb2ddc4.png)


![L-Prolinamide, N-[3-[2-(2-aminoethoxy)ethoxy]-1-oxopropyl]-3-methyl-L-valyl-4-hydroxy-N-[[4-(4-methyl-5-thiazolyl)phenyl]methyl]-, (4R)- HCl ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/412/427/7c030a54-6d2b-4b19-8e4a-a821d42b1d1f/640/7c030a54-6d2b-4b19-8e4a-a821d42b1d1f.png)