Kluczowe dokumenty
S8559
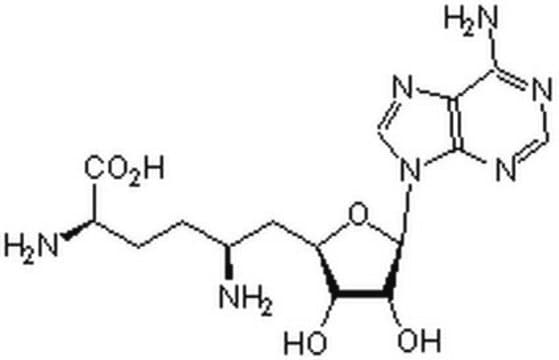
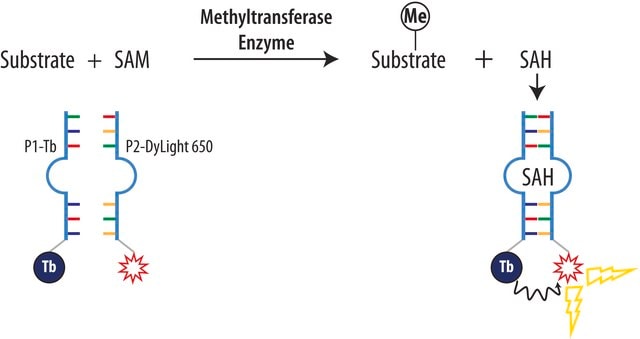
Sinefungin
95% (HPLC), powder, methylation of bases in DNA & RNA inhibitor
Synonim(y):
5′-Deoxy-5′-(1,4-diamino-4-carboxybutyl)adenosine, A-9145, Adenosylornithine, Antibiotic 32232RP
Wybierz wielkość
2550,00 zł
Wybierz wielkość
About This Item
2550,00 zł
Polecane produkty
Nazwa produktu
Sinefungin, 95% (HPLC), powder
Poziom jakości
Próba
95% (HPLC)
Formularz
powder
kolor
white to yellow
rozpuszczalność
H2O: complete 20 mg/ml, clear, colorless to light yellow
H2O: soluble
spektrum działania antybiotyku
neoplastics
Tryb działania
DNA synthesis | interferes
enzyme | inhibits
temp. przechowywania
2-8°C
ciąg SMILES
N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1
Klucz InChI
LMXOHSDXUQEUSF-YECHIGJVSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Methylation inhibition by sinefugin is often accompanied by an altered rate of cytosine deamination that is coupled to transition mutation in the DNA. Sinefugin inhibits Epstein-Barr viral activity and this inhibition is related to the change in DNA methylation and gene expression. It can cause a rate change in several restriction DNA endonuclease activities, including Mme I, which is not connected to the inhibition of the methytransferase activity.
Cechy i korzyści
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Active Filters
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej