C8271
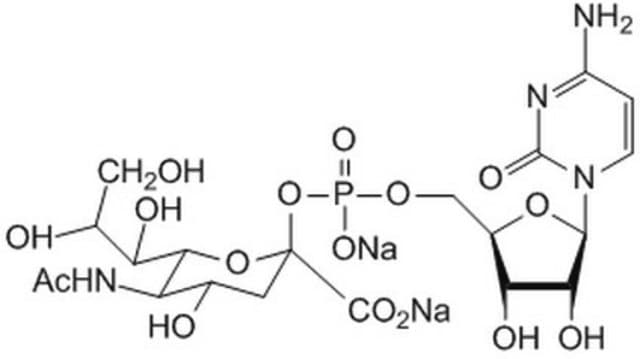
Cytidine-5′-monophospho-N-acetylneuraminic acid sodium salt
≥85% (HPLC)
Synonim(y):
CMP-NAN, CMP-NANA, CMP-Neu5Ac, CMP-Sialic acid
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic (organic)
Próba
≥85% (HPLC)
Postać
powder
temp. przechowywania
−20°C
ciąg SMILES
[Na+].CC(=O)N[C@@H]1[C@@H](O)C[C@@](O[C@@H]1[C@H](O)[C@H](O)CO)(OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N3C=CC(N)=NC3=O)C(O)=O
InChI
1S/C20H31N4O16P.Na/c1-7(26)22-12-8(27)4-20(18(32)33,39-16(12)13(29)9(28)5-25)40-41(35,36)37-6-10-14(30)15(31)17(38-10)24-3-2-11(21)23-19(24)34;/h2-3,8-10,12-17,25,27-31H,4-6H2,1H3,(H,22,26)(H,32,33)(H,35,36)(H2,21,23,34);/q;+1/p-1/t8-,9+,10+,12+,13+,14+,15+,16?,17+,20+;/m0./s1
Klucz InChI
VFRHSOGUONIUOR-CTFMUGKASA-M
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
- as a standard in high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) for nucleotide sugar analysis in Joubert syndrome type 10 (JBTS10) patient cells and control skin fibroblasts,
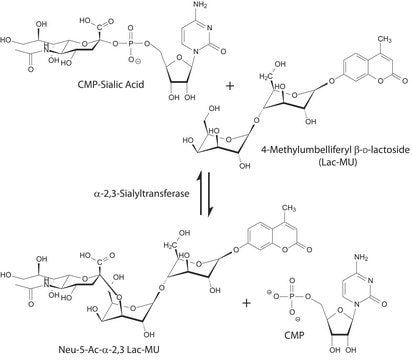
- As a substrate for the enzymatic sialylation of G2 glycoforms, resialylation assay,
- in in-vitro sialyltransferase assay
Działania biochem./fizjol.
Uwaga dotycząca przygotowania
CMP-NAN is very acid-labile.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
LC-MS/MS method quantifies similar polar nucleotide activated sugars using Supel™ Carbon LC column for simultaneous analysis.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Enzymatic glycosyltransferase specificity challenges the one enzyme-one linkage concept.
Understand sialic acid structure, function, signaling, and modifications. Easily find products for sialic acid research.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej