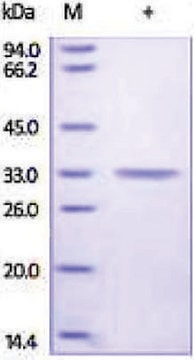
This product is a mixture of the CA-I and CA-I isozymes. The ratio has not been determined.
Kluczowe dokumenty
C3934
Carbonic Anhydrase from bovine erythrocytes
lyophilized powder, ≥2,000 W-A units/mg protein
Synonim(y):
Carbonate Dehydratase, Carbonate Hydrolyase
Wybierz wielkość
1940,00 zł
Wybierz wielkość
About This Item
1940,00 zł
Polecane produkty
pochodzenie biologiczne
bovine erythrocytes
Formularz
lyophilized powder
aktywność właściwa
≥2,000 W-A units/mg protein
masa cząsteczkowa
30 kDa
Zastosowanie
diagnostic assay manufacturing
temp. przechowywania
2-8°C
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Działania biochem./fizjol.
Definicja jednostki
Inhibitor
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Resp. Sens. 1
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Protokoły
Enzymatic Assay of Carbonic Anhydrase for Wilbur-Anderson Units (EC 4.2.1.1)
-
Dear who is concern, I would like to know which isoform of carbonic anhydrase this is.
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the molecular weight of Product C3934, Carbonate Dehydratase?
1 answer-
Because there are a number of closely related isoforms, the molecular weight typically used is approximately 30 kDa. This product is expected to be a mixture of isoforms.
Helpful?
-
-
What is the purity of Product C3934, Carbonate Dehydratase?
1 answer-
Since C3934 is a mixture of isoforms, no purity claim is made. The product is packaged and offered based on its enzymatic activity.
Helpful?
-
-
Can stock solutions of Product C3934, Carbonate Dehydratase, be made and stored?
1 answer-
Carbonic anhydrase solutions can be prepared in water or buffer at concentrations up to 10 mg/mL. It is common for there to be some particulates or haziness in the solution. No data on solution stability has been collected by Sigma-Aldrich, so we recommend solutions be prepared fresh for use.
Helpful?
-
Active Filters
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej