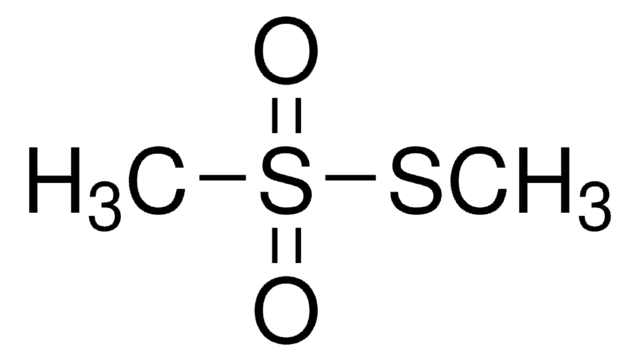
64306
S-Methyl methanethiosulfonate
purum, ≥98.0% (GC)
Synonim(y):
S-Methyl thiomethanesulfonate, MMTS
About This Item
Polecane produkty
klasa czystości
purum
Poziom jakości
Próba
≥98.0% (GC)
współczynnik refrakcji
n20/D 1.513 (lit.)
n20/D 1.513
tw
69-71 °C/0.4 mmHg (lit.)
rozpuszczalność
chloroform: 750mg + 5 ml Chloroform mg/mL, colorless to light greenish-yellow
gęstość
1.337 g/mL at 20 °C
1.337 g/mL at 25 °C (lit.)
temp. przechowywania
2-8°C
ciąg SMILES
CSS(C)(=O)=O
InChI
1S/C2H6O2S2/c1-5-6(2,3)4/h1-2H3
Klucz InChI
XYONNSVDNIRXKZ-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- Modification of Thiol Enzymes: S-methyl methanethiosulfonate (MMTS) offers a unique method for the modification of thiol enzymes and redox-regulated proteins, providing potential applications in biochemical research focused on enzyme regulation and redox biology (Makarov et al., 2019).
- Sensor Development for Protease Activity: S-methyl methanethiosulfonate is used as a blocking reagent on the structural transitions of papain-like cysteine proteases, which supports its utility in sensor development, allowing for the detection and analysis of protease activity in various biological processes (Markovic et al., 2023).
- Agricultural Pathogen Control: Research evaluating S-methyl methanethiosulfonate as a late blight inhibitor highlights its potential as a broad-range toxin against plant pathogens, suggesting applications in agriculture for the management of crop diseases (Joller et al., 2020).
Przestroga
Inne uwagi
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
188.6 °F - closed cup
Temperatura zapłonu (°C)
87 °C - closed cup
Środki ochrony indywidualnej
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej