The suitability of this compound for use in FACS has not been determined. This application would need to be validated by the end user. Please see the link below to a publication that may be helpful:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6718257/
Fontos dokumentumok
Q3251
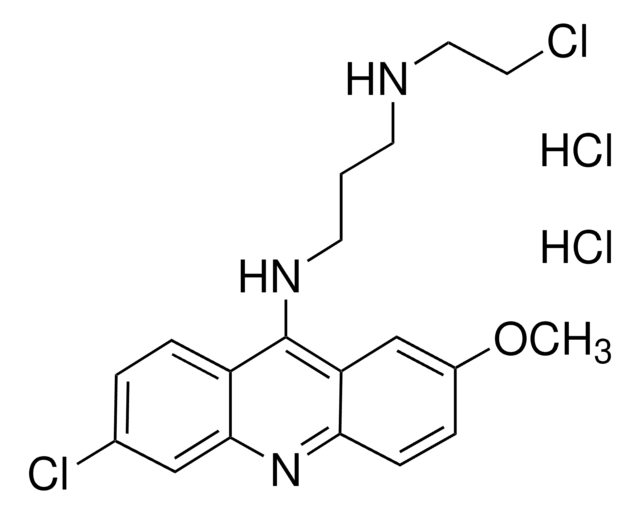
Quinacrine dihydrochloride
≥90% (TLC), powder, MAO-A/B inhibitor
Szinonimák:
6-Chloro-9-(4-diethylamino-1-methylbutylamino)-2-methoxyacridine dihydrochloride, Atebrin dihydrochloride, Mepacrine dihydrochloride
About This Item
Javasolt termékek
Terméknév
Quinacrine dihydrochloride, ≥90%
biológiai forrás
synthetic
Minőségi szint
Teszt
≥90%
Forma
powder
mp
257 °C
oldhatóság
H2O: soluble, clear to hazy
kezdeményező
Bayer
SMILES string
Cl[H].Cl[H].CCN(CC)CCCC(C)Nc1c2ccc(Cl)cc2nc3ccc(OC)cc13
InChI
1S/C23H30ClN3O.2ClH/c1-5-27(6-2)13-7-8-16(3)25-23-19-11-9-17(24)14-22(19)26-21-12-10-18(28-4)15-20(21)23;;/h9-12,14-16H,5-8,13H2,1-4H3,(H,25,26);2*1H
Nemzetközi kémiai azonosító kulcs
UDKVBVICMUEIKS-UHFFFAOYSA-N
Géninformáció
human ... MAOA(4128) , MAOB(4129)
Looking for similar products? Látogasson el ide Útmutató a termékösszehasonlításhoz
Általános leírás
Alkalmazás
Biokémiai/fiziológiai hatások
Tulajdonságok és előnyök
Tárolás és stabilitás
Egyéb megjegyzések
Figyelmeztetés
Warning
Figyelmeztető mondatok
Óvintézkedésre vonatkozó mondatok
Veszélyességi osztályok
Acute Tox. 4 Oral
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Egyéni védőeszköz
dust mask type N95 (US), Eyeshields, Gloves
Válasszon a legfrissebb verziók közül:
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Az ügyfelek ezeket is megtekintették
-
Is it okay to measure fluorescence by using FACS? (with FITC Fluorochrome)
1 answer-
Helpful?
-
-
How long can it be used after purchasing?
1 answer-
This product is not assigned an expiration date or recommended retest date. Products with no expiration date or recommended retest date should be routinely inspected by customers to ensure they perform as expected. These products are also subject to a one-year warranty from the date of shipment. For more information, you may access the Product Dating Information document under ADDITIONAL USEFUL DOCUMENTS ABOUT OUR PRODUCTS at the bottom of the Quality Services page with this link: https://www.sigmaaldrich.com/US/en/life-science/quality-and-regulatory-management/quality-services.
Helpful?
-
-
I’m planning to make stocks for 10mM. What is the storage temperature? Is it same with the product?
1 answer-
Stock solutions are stable for up to 60 h at 20°C.
Helpful?
-
-
What is the storage temperature?
1 answer-
Product Q3251, Quinacrine dihydrochloride, is stored at room temperature.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
Can Product Q3251, Quinacrine dihydrochloride be used for staining platelets?
1 answer-
We have not tested Product Q3251, Quinacrine dihydrochloride for staining platelets. The reference link indicates that platelets can be visualized with the fluorescent dye mepacrine (quinacrine dihydrochloride) at a final concentration of 10 μM. Brian Savage, et al., PNAS, 99 (1): 425-430, (2002).
Helpful?
-
Active Filters
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással