Fontos dokumentumok
P7605
Anti-PARP antibody produced in rabbit
affinity isolated antibody, buffered aqueous solution
Szinonimák:
Anti-Poly[ADP-ribose] Polymerase
About This Item
Javasolt termékek
biológiai forrás
rabbit
Minőségi szint
konjugátum
unconjugated
antitest forma
affinity isolated antibody
antitest terméktípus
primary antibodies
klón
polyclonal
form
buffered aqueous solution
molekulatömeg
antigen 116 kDa
faj reaktivitás
human
technika/technikák
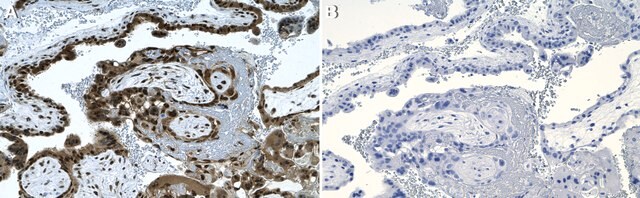
indirect immunofluorescence: 1:100 using cultured MCF7 cells
microarray: suitable
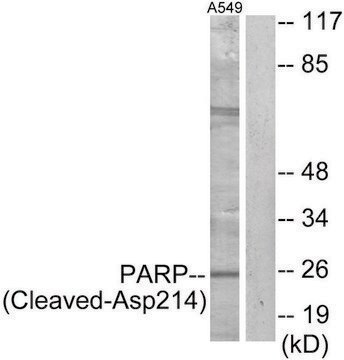
western blot: 1:200 using MCF7 human mammary adenocarcinoma cell extract
UniProt elérési szám
kiszállítva
dry ice
tárolási hőmérséklet
−20°C
célzott transzláció utáni módosítás
unmodified
Géninformáció
human ... PARP1(142)
Általános leírás
Egyediség
Immunogen
Alkalmazás
Biokémiai/fiziológiai hatások
Fizikai forma
Jogi nyilatkozat
Nem találja a megfelelő terméket?
Próbálja ki a Termékválasztó eszköz. eszközt
javasolt
Tárolási osztály kódja
10 - Combustible liquids
WGK
nwg
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Egyéni védőeszköz
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással