129445
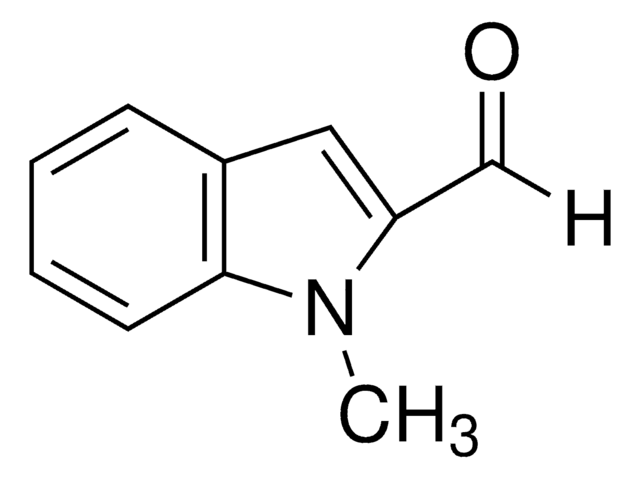
Indole-3-carboxaldehyde
97%
Synonyma:
β-Indolylaldehyde, 3-Formylindole, 3-Indolylformaldehyde, Indole-3-carbaldehyde, NSC 10118
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Empirický vzorec (Hillův zápis):
C9H7NO
Číslo CAS:
Molekulová hmotnost:
145.16
Beilstein/REAXYS Number:
114117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
assay
97%
form
solid
mp
193-198 °C (lit.)
functional group
aldehyde
SMILES string
O=Cc1c[nH]c2ccccc12
InChI
1S/C9H7NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-6,10H
InChI key
OLNJUISKUQQNIM-UHFFFAOYSA-N
Hledáte podobné produkty? Navštivte Průvodce porovnáváním produktů
General description
Indole-3-carboxaldehyde can undergo Schiff bases condensation to form multifunctional silica nano-vehicles and magnetic nanoparticles.
Application
Indole-3-carboxaldehyde was used to prepare analogs of the indole phytoalexin cyclobrassinin with NR1R2 group. It was also used as the starting material for the synthesis of higher order indoles including isoindolo[2,1-a]indoles, aplysinopsins, and 4-substituted-tetrahydrobenz[cd]indoles.
Reactant for preparation of:
- Analgesic agents
- Hypoglycemic agents
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Antibacterial and antifungal agents
- Antiamoebic and cytotoxic agents
- Inhibitors of the Dengue virus protease with antiviral activity in cell-culture
- Curcumin analogues as possible anti-proliferative & anti-inflammatory agents
- Inhibitors of Bcl-2 family proteins
- Inhibitors of the C-terminal domain of RNA Polymerase II as antitumor agents
- Inhibitors of TNF-α and IL-6 with anti-tubercular activity
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Qiu-Yun Chen et al.
Colloids and surfaces. B, Biointerfaces, 114, 158-163 (2013-11-05)
Multifunctional silica nano-vehicles (SiO2@indol-IL) and magnetic nanoparticles (Fe3O4@indol-IL) were constructed through the Schiff bases condensation of indole-3-carboxaldehyde and 4-acetyl-N-allyl pyridinium chloride (ILs) with the amine groups of silica and magnetic nanoparticles. SiO2@indol-IL can inhibit the proliferation of HepG-2 cells in
Mariana Budovská et al.
Bioorganic & medicinal chemistry, 21(21), 6623-6633 (2013-09-10)
An effective synthesis of analogs of the indole phytoalexin cyclobrassinin with NR1R2 group instead of SCH3 was developed starting from indole-3-carboxaldehyde. The target compounds were prepared by spirocyclization of 1-Boc-thioureas with the formation of isolable spiroindoline intermediates, followed by the
Heterocycles, 38, 1479-1479 (1994)
Indian J. Chem. B, 33, 4-4 (1994)
Anna M Vetrano et al.
The Journal of biological chemistry, 280(42), 35372-35381 (2005-08-05)
Catalase is a highly conserved heme-containing antioxidant enzyme known for its ability to degrade hydrogen peroxide into water and oxygen. In low concentrations of hydrogen peroxide, the enzyme also exhibits peroxidase activity. We report that mammalian catalase also possesses oxidase
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.