69899
Monochlorobimane
suitable for fluorescence, ≥70.0% (HPCE)
Synonim(y):
mBCl, Chlorobimane
About This Item
Polecane produkty
Poziom jakości
Próba
≥70.0% (HPCE)
Postać
powder
mp
135-136 °C (lit.)
rozpuszczalność
DMF: soluble
DMSO: soluble
acetonitrile: soluble
methanol: soluble
fluorescencja
λex 380 nm; λem 461 nm in methanol
λex 390 nm; λem 478 nm in 0.1 M phosphate pH 7.5 (after derivatization with glutathione)
przydatność
suitable for fluorescence
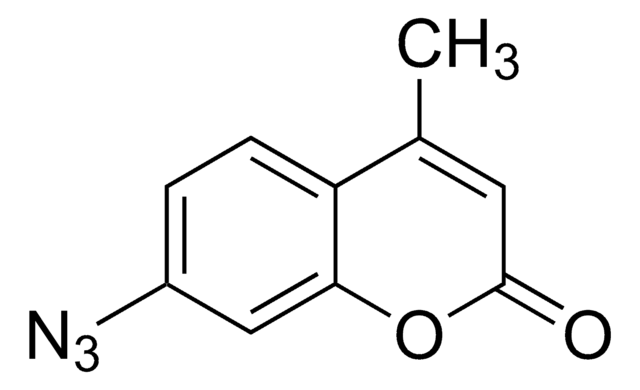
ciąg SMILES
CC1=C(C)C(=O)N2N1C(CCl)=C(C)C2=O
InChI
1S/C10H11ClN2O2/c1-5-7(3)12-8(4-11)6(2)10(15)13(12)9(5)14/h4H2,1-3H3
Klucz InChI
SUIPVTCEECPFIB-UHFFFAOYSA-N
Opis ogólny
Monochlorobimane, also known as mBCl, is a non-fluorescent compound that forms a fluorescent complex upon reaction. The fluorescence is detected at 394/490nm.
Zastosowanie
Opakowanie
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Fluorescence lifetime measurement is advantageous over intensity-based measurements. Applications include fluorescence lifetime assays, sensing and FLI.
Pomiar czasu życia fluorescencji ma przewagę nad pomiarami opartymi na intensywności. Zastosowania obejmują testy czasu życia fluorescencji, wykrywanie i FLI.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej