706531
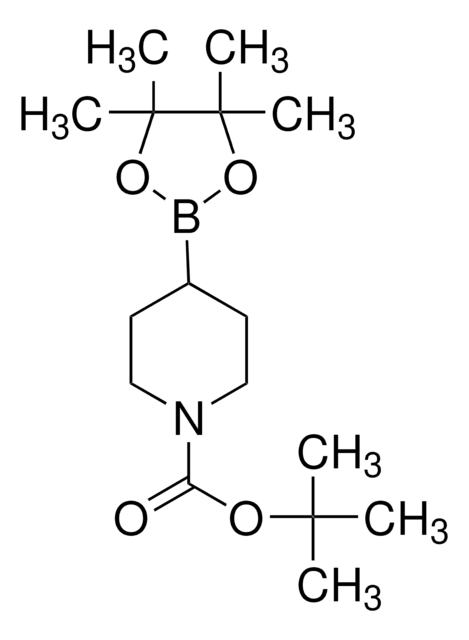
N-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
95%
Sinonimo/i:
(1-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,3,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)boronic acid pinacol ester, (N-tert-Butoxycarbonyl)-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester
Scegli un formato
68,00 €
Prezzo di listino136,00 €Risparmia il 50%Scegli un formato
About This Item
68,00 €
Prezzo di listino136,00 €Risparmia il 50%Prodotti consigliati
Livello qualitativo
Saggio
95%
Stato
powder
Punto di fusione
100-114 °C
Stringa SMILE
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
VVDCRJGWILREQH-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Applicazioni
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst[1]
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling[2]
- Palladium-catalyzed Suzuki-Miyaura coupling[3]
- Suzuki coupling followed by iodolactonization reaction[4]
- Wrenchnolol derivative optimized for gene activation in cells[5]
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors[6]
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes[7]
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands[8]
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder[9]
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists[10]
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

