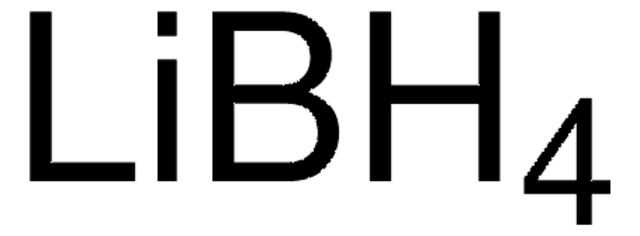If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
230200
Lithium borohydride solution
2.0 M in THF
Sinonimo/i:
Lithium boron hydride, Lithium hydroborate
About This Item
Prodotti consigliati
Stato
liquid
Livello qualitativo
Impiego in reazioni chimiche
reagent type: reductant
Concentrazione
2.0 M in THF
Densità
0.896 g/mL at 25 °C
Stringa SMILE
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
UUKMSDRCXNLYOO-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes
- Mechano-chemical metathesis reactions
- Noncatalytic hydrolysis for hydrogen generation
- Growth of large gold monolayer protected-clusters
- Anion substitution reactions
- Dehydrogenation reactions
Confezionamento
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Organi bersaglio
Central nervous system, Respiratory system
Rischi supp
Codice della classe di stoccaggio
4.3 - Hazardous materials which set free flammable gases upon contact with water
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
-0.4 °F - closed cup
Punto d’infiammabilità (°C)
-18 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
-
How can I determine the shelf life / expiration / retest date of this product?
1 risposta-
Utile?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 risposta-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Utile?
-
-
Is the tetrahydrofuran (THF) used in Product 230200, Lithium Borohydride, stabilized?
1 risposta-
The THF used in this product is stabilized with 0.025% butylated hydroxytoluene (BHT).
Utile?
-
-
Is Product 230200, Lithium Borohydride, an energy carrier?
1 risposta-
Lithium borohydride is renowned for one of the highest energy density chemical energy carriers. By reacting with atmospheric oxygen, it liberates large amounts of heat.
Utile?
-
-
What is the Department of Transportation shipping information for this product?
1 risposta-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Utile?
-
-
What types of reactions is Product 230200, Lithium Borohydride, used in?
1 risposta-
It is reagent for reduction of compounds containing ketonic, aldehydic, or ester carbonyls and a nitrile group, where reduction of the carbonyl but not of the nitrile group is wanted.
Utile?
-
-
Why does Product 230200, Lithium Borohydride, need to be handled under nitrogen?
1 risposta-
This product is moisture sensitive, reacting violently with water, liberating extremely flammable gases.
Utile?
-
-
Is it necessary to refrigerate Product 230200, Lithium Borohydride?
1 risposta-
We do recommend to refrigerate this product, since it may develop pressure at room temperature.
Utile?
-
Filtri attivi
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.