Fontos dokumentumok
SML1949
Synta66
≥98% (HPLC)
Szinonimák:
4-Pyridinecarboxamide, N-(2′,5′-dimethoxy[1,1′-biphenyl]-4-yl)-3-fluoro-, GSK1349571A, N-(2′,5′-Dimethoxy[1,1′-biphenyl]-4-yl)-3-fluoro-4-pyridinecarboxamide, S66, Synta 66, Synta-66
About This Item
Javasolt termékek
Minőségi szint
Teszt
≥98% (HPLC)
form
powder
szín
white to beige
oldhatóság
DMSO: 20 mg/mL, clear
tárolási hőmérséklet
2-8°C
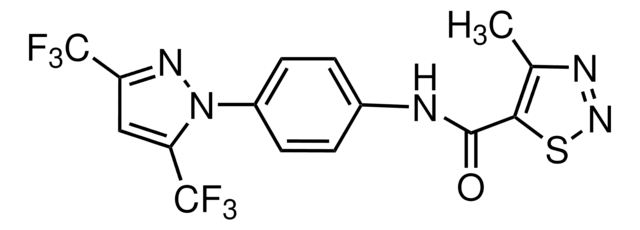
SMILES string
O=C(NC1=CC=C(C2=C(OC)C=CC(OC)=C2)C=C1)C3=CC=NC=C3F
Alkalmazás
- as a Ca2+ release-activated calcium (CRAC) channel inhibitor to study its effects on ORAI isoforms
- as an ORAI1 blocker to study its effects on the entry of Ca2+ in chronic lymphocytic leukemia (CLL) B cells
- as a CRAC blocker to study its effects on the influx of Ca2+ by store-operated Ca2+ entry (SOCE) in enamel cells
Biokémiai/fiziológiai hatások
Tárolási osztály kódja
11 - Combustible Solids
WGK
WGK 3
Lobbanási pont (F)
Not applicable
Lobbanási pont (C)
Not applicable
Analitikai tanúsítványok (COA)
Analitikai tanúsítványok (COA) keresése a termék sarzs-/tételszámának megadásával. A sarzs- és tételszámok a termék címkéjén találhatók, a „Lot” vagy „Batch” szavak után.
Már rendelkezik ezzel a termékkel?
Az Ön által nemrégiben megvásárolt termékekre vonatkozó dokumentumokat a Dokumentumtárban találja.
Tudóscsoportunk valamennyi kutatási területen rendelkezik tapasztalattal, beleértve az élettudományt, az anyagtudományt, a kémiai szintézist, a kromatográfiát, az analitikát és még sok más területet.
Lépjen kapcsolatba a szaktanácsadással