497401
(R)-(+)-2-Methyl-2-propanesulfinamide
98%
Synonyma:
(R)-2-methyl-2-propanesulfinamide, (R)-2-methylpropane-2-sulfinamide, (R)-tert-butanesulfinamide, (R)-tert-butylsulfinamide
About This Item
Doporučené produkty
assay
98%
optical activity
[α]20/D +4°, c = 1.0242 in chloroform stab. with amylenes
mp
103-107 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m1/s1
InChI key
CESUXLKAADQNTB-SSDOTTSWSA-N
Související kategorie
General description
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Sortimentní položky
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Související obsah
Ellman group developed electron-rich phosphine ligands for C-H functionalization and tert-Butanesulfinamide for asymmetric amine synthesis.
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.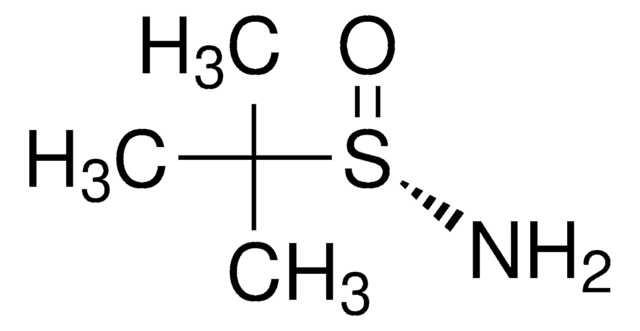








![(R)-N-[(1R,2R)-2-(3-(3,5-Bis(trifluoromethyl)phenyl)ureido)cyclohexyl]-tert-butyl-sulfinamide 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)