497312
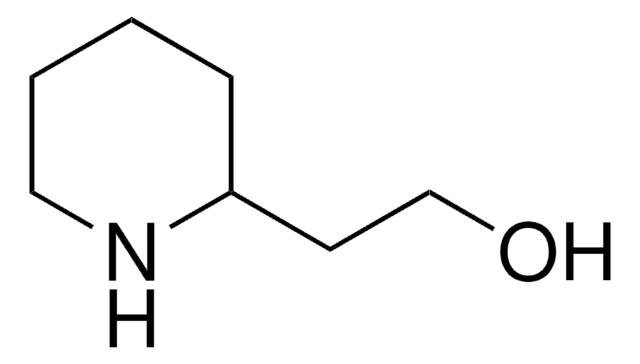
4-Piperidinemethanol
97%
Synonyma:
4-(Hydroxymethyl)piperidine
Přihlásitk zobrazení cen stanovených pro organizaci a smluvních cen
About This Item
Empirický vzorec (Hillův zápis):
C6H13NO
Číslo CAS:
Molekulová hmotnost:
115.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Doporučené produkty
Quality Level
assay
97%
bp
118-120 °C/10 mmHg (lit.)
mp
55-59 °C (lit.)
functional group
hydroxyl
SMILES string
OCC1CCNCC1
InChI
1S/C6H13NO/c8-5-6-1-3-7-4-2-6/h6-8H,1-5H2
InChI key
XBXHCBLBYQEYTI-UHFFFAOYSA-N
Související kategorie
General description
4-Piperidinemethanol is a cyclic secondary amine. Its standard molar enthalpies of combustion, sublimation and formation have been determined.
Application
4-Piperidinemethanol may be used in the preparation of:
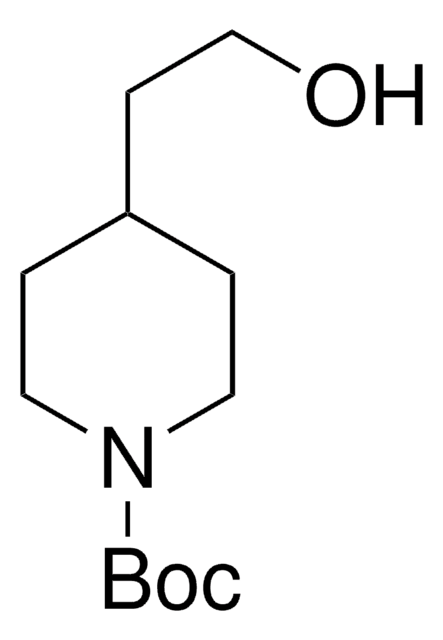
- N-tert-butoxycarbonyl-4-hydroxymethyl piperidine
- desferrioxamine B (DFO) containing third generation triazine dendrimer
- ethyl 3-(4-(hydroxymethyl)piperidin-1-yl)propanoate (EHMPP)
- 4-(hydroxymethyl)piperidine-1-carbodithioic acid (HL)
- 1-[[(1E)-2-(4-chlorophenyl)ethenyl]sulfonyl]-4-piperidinemethanol
Substrate used in solid-phase organic synthesis of a secondary amine.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Vyberte jednu z posledních verzí:
Již tento produkt vlastníte?
Dokumenty související s produkty, které jste v minulosti zakoupili, byly za účelem usnadnění shromážděny ve vaší Knihovně dokumentů.
Zákazníci si také prohlíželi
Jongdoo Lim et al.
Bioorganic & medicinal chemistry, 18(15), 5749-5753 (2010-07-10)
The synthesis of a third generation triazine dendrimer, 1, containing multiple, iron-sequestering desferrioxamine B (DFO) groups is described. Benzoylation of the hydroxamic acid groups of DFO and formation of a reactive dichlorotriazine provide the intermediate for reaction with the second
Synthesis, spectroscopy, and biological activity of heterobimetallic complexes containing Sn (IV) and Pd (II) with 4-(hydroxymethyl) piperidine-1-carbodithioic acid.
Anwar MT, et al.
Russ. J. Gen. Chem., 83(12), 2380-2385 (2013)
Christian A Olsen et al.
Organic letters, 6(12), 1935-1938 (2004-06-05)
[reaction: see text] An expedient solid-phase synthetic approach to secondary and tertiary amines was developed. The protocol employs conversion of resin-bound amino alcohols to the corresponding iodides, followed by iodide displacement with primary or secondary amines or with unprotected amino
Francis Giraud et al.
Bioorganic & medicinal chemistry letters, 19(2), 301-304 (2008-12-19)
Continuous efforts on the synthesis and structure-activity relationships (SARs) studies of modified 1-benzylamino-2-phenyl-3-(1H-1,2,4-triazol-1-yl)propan-2-ols as antifungal agents, allowed identification of new 1-[(pyridinyl- and piperidinylmethyl)amino] derivatives with MIC(80) values ranging from 1410.0 to 23.0ngmL(-1) on Candidaalbicans. These results confirmed both the importance
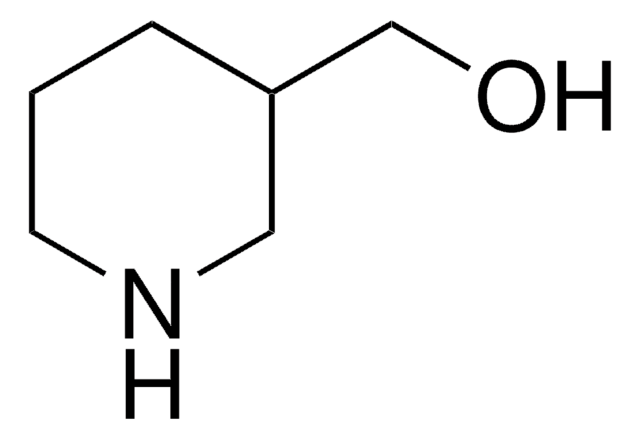
Standard molar enthalpies of formation of 2-, 3-, and 4-piperidinomethanol isomers.
da Silva MAVR and Cabral JITA
The Journal of Chemical Thermodynamics, 38(8), 1008-1012 (2006)
Náš tým vědeckých pracovníků má zkušenosti ve všech oblastech výzkumu, včetně přírodních věd, materiálových věd, chemické syntézy, chromatografie, analytiky a mnoha dalších..
Obraťte se na technický servis.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)



