PHR1258
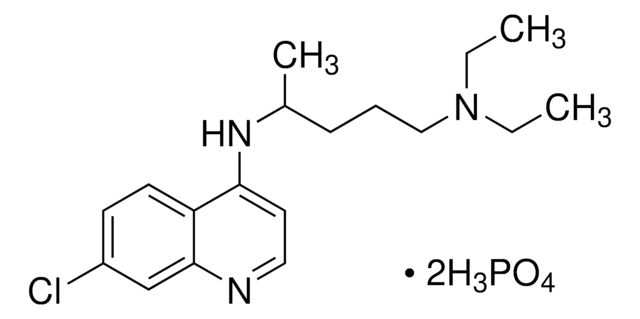
Chloroquin -diphosphat (Salz)
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(e):
N4-(7-Chlor-4-chinolinyl)-N1,N1-diethyl-1,4-pentandiamin -diphosphat (Salz)
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
certified reference material
pharmaceutical secondary standard
Agentur
BP
EP
USP
traceable to USP 1118000
Dampfdruck
<0.0000001 kPa ( 25 °C)
API-Familie
chloroquine
Analysenzertifikat (CofA)
current certificate can be downloaded
Verpackung
pkg of 1 g
Lagerbedingungen
protect from light
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
Farbe
white to yellow
pH-Wert
3.5-4.5 (10% in aqueous solution, pH <6.0)
mp (Schmelzpunkt)
200 °C (dec.) (lit.)
Löslichkeit
water: 50 g/L
benzene: practically insoluble
chloroform: practically insoluble
ethanol: practically insoluble
ether: practically insoluble
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-8°C
SMILES String
OP(O)(O)=O.OP(O)(O)=O.CCN(CC)CCCC(C)Nc1ccnc2cc(Cl)ccc12
InChI
1S/C18H26ClN3.2H3O4P/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18;2*1-5(2,3)4/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21);2*(H3,1,2,3,4)
InChIKey
QKICWELGRMTQCR-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Hinweis zur Analyse
Sonstige Hinweise
Fußnote
Empfohlene Produkte
Ähnliches Produkt
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.