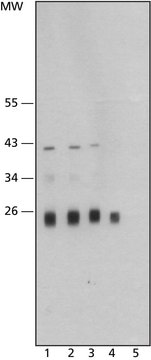
C6854
Cathepsin L from human liver
≥0.5 units/mg protein, solution
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Número CAS:
Número MDL:
Código UNSPSC:
12352200
NACRES:
NA.32
Produtos recomendados
fonte biológica
human liver
Nível de qualidade
forma
solution
atividade específica
≥0.5 units/mg protein
técnica(s)
activity assay: suitable
faixa de pH
4.5—5.5
nº de adesão UniProt
aplicação(ões)
pharmaceutical
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
Informações sobre genes
human ... CTSL1(1514)
Descrição geral
The cathepsin L (CTSL) gene is mapped to human chromosome 9q21.33. It encodes a lysosomal cysteine proteinase that belongs to the peptidase C1 family. The enzyme is a dimer of a heavy (175 amino acids) and a light chain (42 amino acids) linked by disulfide bonds.
Aplicação
Cathepsin L (CTSL) from human liver has been used:
- to digest mutant transforming growth factor β-induced (TGFBIp) and LC3 (autophagosomes marker) proteins in vitro
- to digest the pH-dependent fibril of a pre-melanosomal protein (Pmel17) repeat domain (RPT) isoform and study the effect of pH on sRPT aggregation kinetics by tryptophan fluorescence
- to analyze the inhibition of CTSL by small molecules or drugs in a cell-free system
Ações bioquímicas/fisiológicas
Cathepsin L (CTSL) exhibits endopeptidase activity and breaks peptide bonds with aromatic residues. This enzyme is functional at pH 3.0–6.5 with thiol compounds. Elastin, collagen, and α-1 protease inhibitors act as substrates for cathepsin L (CTSL). CTSL cleaves several proteins including enzymes, receptors, and transcription factors. It is involved in antigenic peptide production and controls B-cells homeostasis. It is associated with myofibril necrosis and tumor progression. Increased expression of CTSL promotes metastasis and relates to poor prognosis in cancer patients. Increased expression of CTSL is observed in breast cancer. CTSL is implicated in glioblastoma multiforme (GBM), middle east respiratory syndrome (MERS), gingival overgrowth, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has a higher specific activity than cathepsin B and H in the degradation of a variety of physiological protein substrates.
Definição da unidade
One unit will hydrolyze 1.0 μmole of Z-Phe-Arg-AFC per minute at pH 5.5 at 25 °C.
forma física
Solution in in 20 mM malonate, pH 5.5, 1 mM EDTA, and 400 mM NaCl.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Stefan Tholen et al.
Cellular and molecular life sciences : CMLS, 71(5), 899-916 (2013-07-03)
Endolysosomal cysteine cathepsins functionally cooperate. Cathepsin B (Ctsb) and L (Ctsl) double-knockout mice die 4 weeks after birth accompanied by (autophago-) lysosomal accumulations within neurons. Such accumulations are also observed in mouse embryonic fibroblasts (MEFs) deficient for Ctsb and Ctsl.
M Kathryn Liszewski et al.
Immunity, 39(6), 1143-1157 (2013-12-10)
Complement is viewed as a critical serum-operative component of innate immunity, with processing of its key component, C3, into activation fragments C3a and C3b confined to the extracellular space. We report here that C3 activation also occurred intracellularly. We found
Dexter N Dean et al.
Biochimica et biophysica acta. Proteins and proteomics, 1867(10), 961-969 (2019-02-05)
The pre-melanosomal protein (Pmel17) aggregates within melanosomes to form functional amyloid fibrils that facilitate melanin polymerization. The repeat domain (RPT) of Pmel17 fibrillates under strict acidic melanosomal pH. Alternative splicing results in a shortened repeat domain (sRPT), which also forms
Gustavo E Chavarria et al.
European journal of medicinal chemistry, 58, 568-572 (2012-11-22)
Kinetic analysis of the mode of inhibition of cathepsin L by KGP94, a lead compound from a privileged library of functionalized benzophenone thiosemicarbazone derivatives, demonstrated that it is a time-dependent, reversible, and competitive inhibitor of the enzyme. These results are
Bailey Miller et al.
Journal of natural products, 77(1), 92-99 (2013-12-25)
A number of marine natural products are potent inhibitors of proteases, an important drug target class in human diseases. Hence, marine cyanobacterial extracts were assessed for inhibitory activity to human cathepsin L. Herein, we have shown that gallinamide A potently
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica